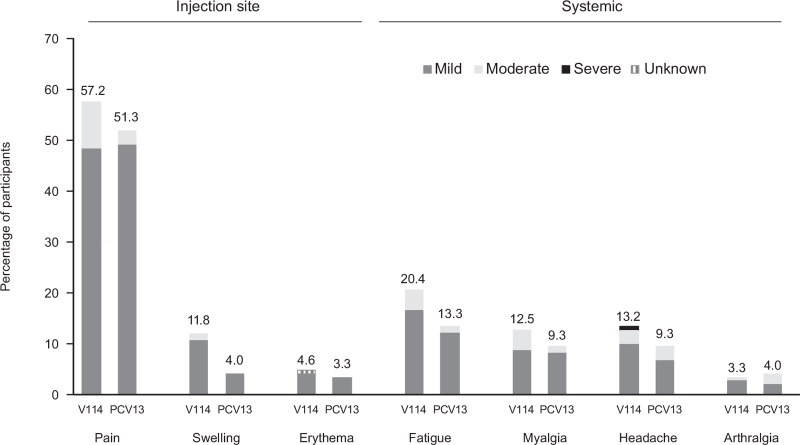
Fig. 2.
Proportions of participants with solicited adverse events after vaccination with V114 or PCV13 by severity.
Solicited adverse events collected postvaccination (days 1–5 for injection-site events and days 1–14 for systemic events) are shown with severity grades (V114: n = 152; PCV13: n = 150). The height of the stacked bar represents the total percentage of participants reporting the adverse event. The severity grades (mild, moderate, or severe) within the bar indicate the proportion of the total attributed to each respective category. PCV13, 13-valent pneumococcal conjugate vaccine; V114, 15-valent pneumococcal conjugate vaccine.