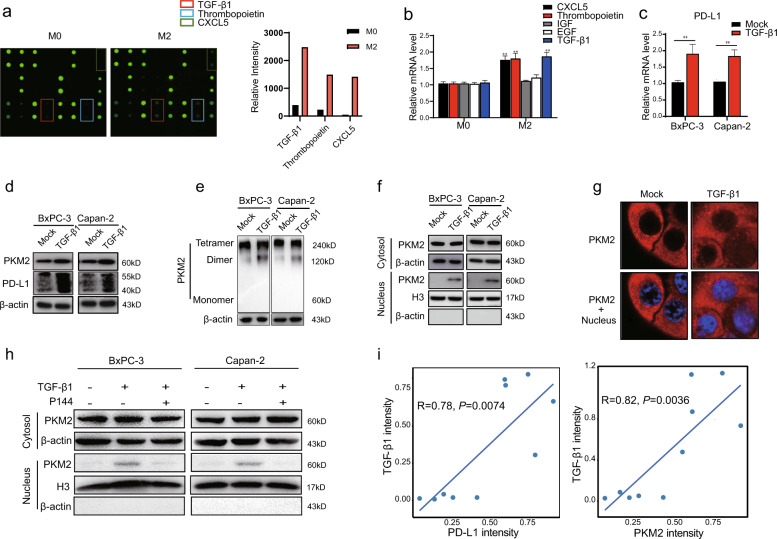
Fig. 4. TGF-β1 secreted by M2 macrophages promotes the nuclear translocation of PKM2 in PDAC cells.
a Cytokine antibody arrays were performed in the culture media of M0/M2 macrophages co-cultured with BxPC-3 cells (left). The relative signal intensity of indicated cytokines in M2 macrophages co-cultured with BxPC-3 cells compared with M0 macrophages co-cultured BxPC-3 cells (right). b Quantitative RT-PCR analysis of mRNA levels of CXCL5, Thrombopoietin, IGF, EGF, and TGF-β1 in M0/M2 macrophages co-cultured with BxPC-3/Capan-2 cells for 48 h. c–g BxPC-3/Capan-2 cells were treated with or without TGF-β1 (20 ng/mL). c Quantitative RT-PCR analysis of mRNA expression levels of PD-L1 in BxPC-3/Capan-2 cells. d PKM2 and PD-L1 protein expression levels in BxPC-3/Capan-2 cells were determined using western blotting. e BxPC-3/Capan-2 cells were cross-linked by glutaraldehyde first and then subjected to western blotting. f Nuclear and cytosolic lysates were prepared from BxPC-3/Capan-2 cells, followed by western blotting. β-actin and histone H3 were used as loading controls. g The subcellular localization of PKM2 in BxPC-3/Capan-2 cells. Cells were immunostained with anti-PKM2 (PKM2, red). The nucleus was marked with 4′,6-diamidino-2-phenylindole dihydrochloride (blue). The stained cells were imaged using the ZEN 3.0 (Carl Zeiss) software. h BxPC-3/Capan-2 cells were treated with or without TGF-β1 (20 ng/mL), and P144 (10 μM) was used to block TGF-β1. Nuclear and cytosolic lysates were prepared from BxPC-3/Capan-2 cells, followed by western blotting. i Correlation analyses between the expression of TGF-β1 and PKM2/PD-L1 in PDAC tissues according to western blotting (n = 10). Data were shown as mean ± SEM from three experiments. Statistical significance was determined by a t-test; *P < 0.05,**P < 0.01,***P < 0.001.