Figure 2. High rates of initial remission and relapse in pediatric patients treated with tisagenlecleucel.
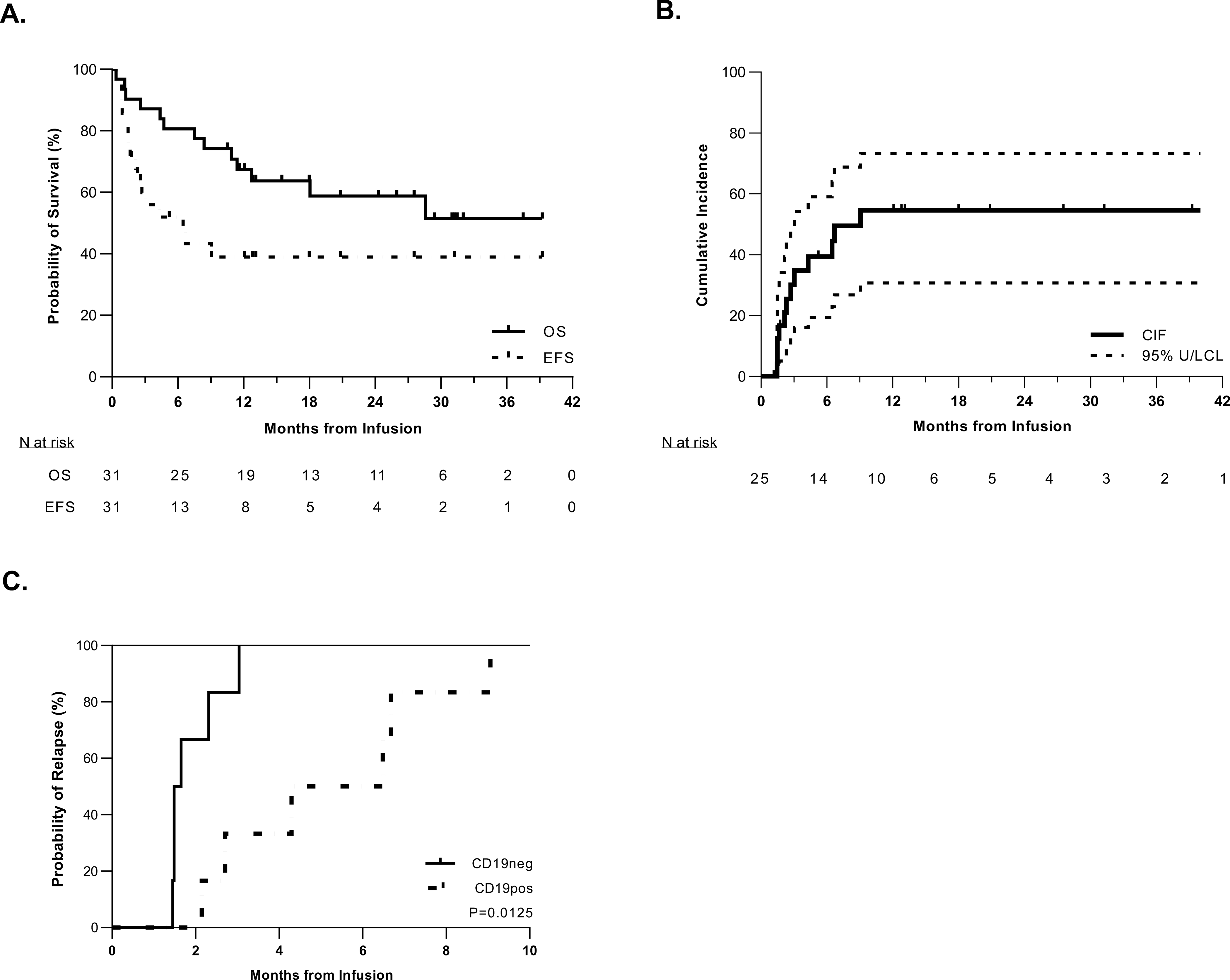
A. Kaplan-Meier curve of event-free survival (EFS) and overall survival (OS) in the total cohort of 31 patients. The median time of follow-up was 329 days (range: 11–1046 days). The median EFS time was 4.3 months (95%CI: 1.5-NA) (NA = not able to be calculated); the median OS has not been reached but the last death occurred at 28.9 months giving a 51.5% OS rate. B. Cumulative incidence of relapse among patients who experienced an initial CR. Relapse was defined as recurrent detectable disease after initial CR, including minimal residual disease (MRD-positive: ≥0.01% blasts by flow cytometry, ≥10−4 by PCR, and/or ≥10−5 by NGS). Death was considered as a competing risk. C. Cumulative incidence of relapse defined by antigen subtype: CD19-positive (n = 6) vs. CD19-negative (n = 6). CD19-negative relapse occurred sooner than CD19-positive relapse, with the median times to occurrence being 1.6 months and 5.4 months post infusion, respectively (P = 0.0125).
