Figure 3. Disease burden of ≥5% before CAR T-cell therapy predicts worse outcomes after tisagenlecleucel treatment.
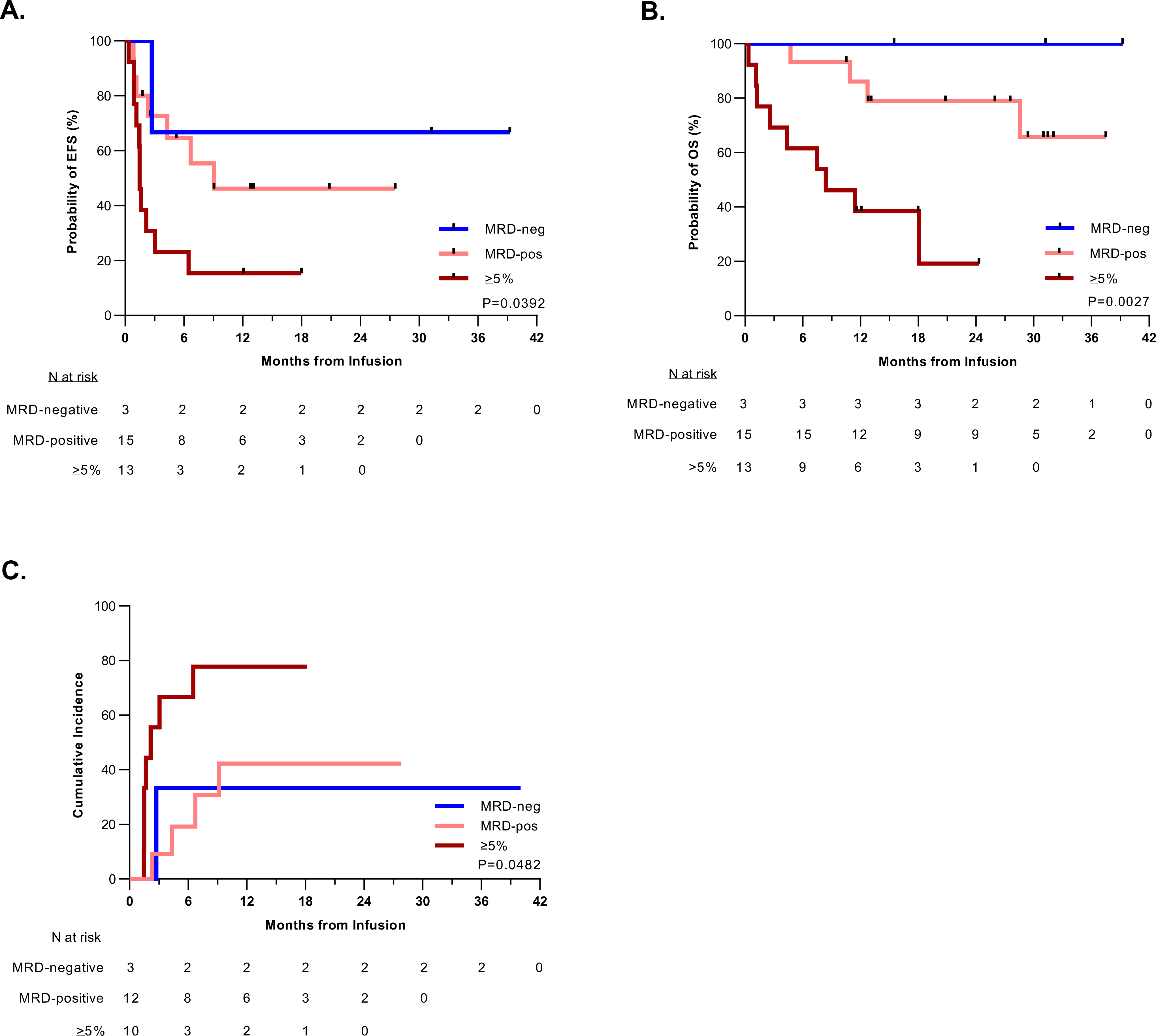
A. Kaplan-Meier estimation of the event-free survival (EFS) of patients based on their pre-infusion disease burden. Patients with higher pretreatment disease burden had worse EFS (P = 0.0392). Median EFS time based on disease burden: ≥5% blasts: 1.5 months (95% CI: 0.9–3.0); MRD-positive (>0-<5% blasts): 9.1 months (95% CI: 1.2-NA); MRD-negative: NA. B. Kaplan-Meier estimation of the overall survival (OS) of patients stratified by pre-infusion disease burden. Patients with higher pretreatment disease burden had worse OS (P = 0.0027). The median survival for patients with a disease burden of ≥5% was 8.4 months (95% CI: 1.3-NA) and unable to be calculated (NA) for patients with pretreatment MRD-positive or MRD-negative disease. C. Cumulative incidence of relapse after initial CR among patients by pre-infusion disease burden, such that patients with a disease burden of ≥5% had a higher rate of relapse (P = 0.048), with a median time to relapse of 2.2 months. The median time to relapse could not be calculated (NA) for patients with pretreatment MRD-positive or MRD-negative disease. Relapse included any recurrent detectable disease, including MRD-positive cases. Death in remission was considered as a competing risk.
