Figure 7. The cell cycle and its molecular interaction with the circadian clock:
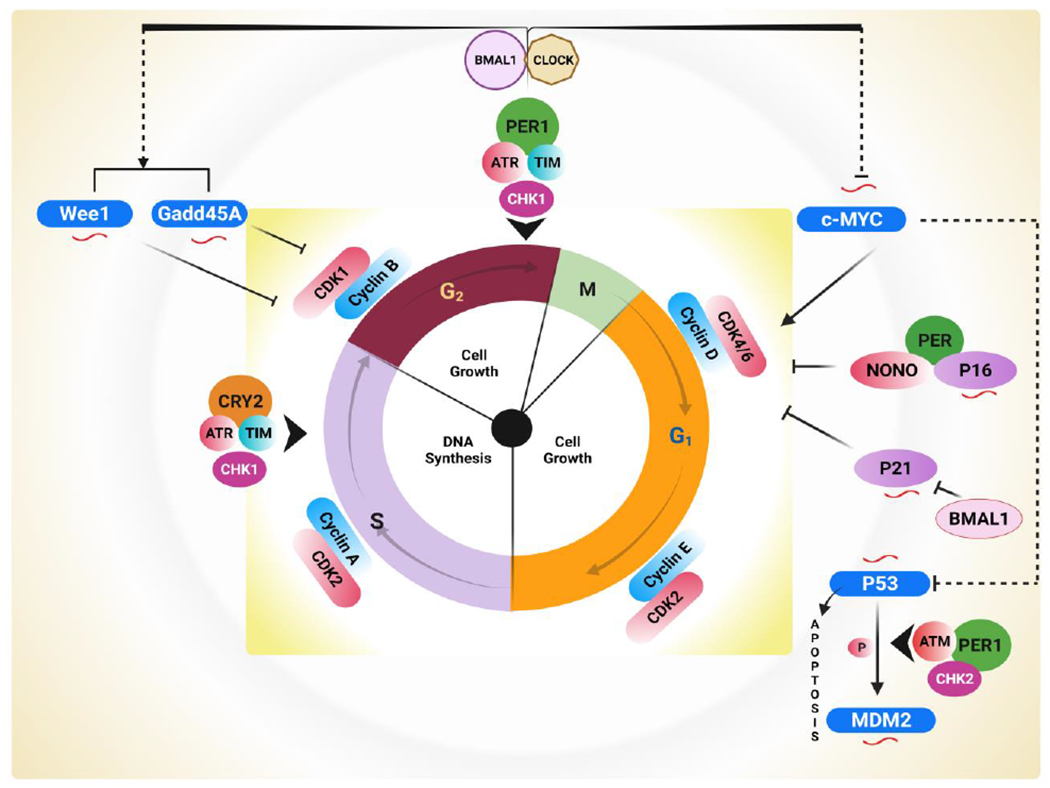
The cell cycle, comprised of several stages regulated by CDK/Cyclin complexes, is involved in the control of the progression of cells. CLOCK transcriptionally governs the expression of Wee1 and Gadd45A proteins. Bmal1, through the CDK1/Cyclin B complex, is involved in the inhibition of the G2/M transition of the cell cycle. Further, activation of the c-Myc oncogene promotes the expression of Cyclin D and cell proliferation. Transcription of the tumor suppressor genes p21 and p16 is under circadian control through checkpoints G1 and G1/S. The circadian output effector NONO connects to p16 and promotes transcription with the association of Per. Another CDK inhibitor, p21, is negatively regulated by BMal1. In addition, the interaction between p53 and Per results in increased activation of p53 target genes, including p21 and mdm2, which are regulated by the ATM–Per1–CHK2 complex. In contrast, ATR–Chk1–CRY–TIM pathways regulate the early response to DNA damage by interacting with Cry and Per clock proteins during the S phase and G2/M checkpoints. NONO: Nuclear RNA II binding protein or GADD45A; p54nrb; Growth Arrest; and DNA Damage inducible 45; TIM: Timeless mammalian protein; CHK2: Checkpoint kinase 2; CHK1: Checkpoint kinase 1; ATR: Ataxia telangiectasia and rad3-related; ATM: Ataxia-telangiectasia mutated. Circadian clock-regulated cell cycle genes are symbolized by ( ). (Created with BioRender.com)
). (Created with BioRender.com)
