Abstract
“Frontal lobe syndrome” is a term often used to describe a diverse array of personality disturbances following frontal lobe damage. This study’s guiding premise was that greater neuroanatomical specificity could be achieved by evaluating specific types of personality disturbances following acquired frontal lobe lesions. We hypothesized that three acquired personality disturbances would be associated with lesion involvement of distinct sectors of the prefrontal cortex (PFC): 1) emotional-social disturbance and ventromedial PFC, 2) hypoemotional disturbance and dorsomedial PFC, and 3) dysexecutive and dorsolateral PFC. In addition, we hypothesized that distressed personality disturbance would not be associated with focal PFC lesions in any sector. Each hypothesis was pre-registered and tested in 182 participants with adult-onset, chronic, focal brain lesions studied with an observational, cross-sectional design. Pre- and postmorbid personality was assessed by informant-rating with the Iowa Scales of Personality Change, completed by a spouse or family member. Two complementary analytic approaches were employed: 1) a hypothesis-driven region-of-interest (ROI) regression analysis examining the associations of lesions in specific PFC sectors with acquired personality disturbances; 2) a data-driven multivariate lesion-behavior mapping analysis, which was not limited to pre-specified regions. Each hypothesis received some support: (i) Emotional/social personality disturbance was most strongly associated with ventromedial PFC lesions in both statistical approaches. (ii) Hypoemotional disturbance was associated with dorsomedial PFC lesions in the ROI analyses, without any significant lesion-symptom mapping associations. (iii) Dysexecutive personality disturbance was associated with bilateral dorsolateral PFC lesions and ventromedial PFC lesions; lesion-symptom mapping showed maximal association of executive dysfunction with damage of the right middle frontal gyrus within the dorsolateral PFC. (iv) Distressed personality disturbance was not associated with lesions in any PFC sector. Altogether, the findings can be interpreted to indicate that damage to different prefrontal sectors may disrupt different anatomical-functional systems and result in distinct personality disturbances.
Keywords: acquired personality disturbance, hypoemotionality, lesion mapping, multivariate lesion-symptom mapping, real-life functioning
1. Introduction
There is a long history of observing personality disturbances following acquired focal brain lesions (Harlow, 1868; Kleist, 1934; Kretschmer, 1956; Logue, Durward, Pratt, Piercy, & Nixon, 1968; Luria, 1969; Phelps, 1897; Rylander, 1939; Walch, 1956). In the current paper, personality refers to enduring tendencies impacting psychosocial functioning across real-life situations; including drive, affect, mood, and cognitive tendencies such as self-awareness, pervasive attitudes, flexibility, judgment and planfulness (Stuss, Gow, & Hetherington, 1992). Despite longstanding interest in personality changes associated with focal brain damage, a detailed understanding of such relationships has remained elusive. Personality disturbances have been reported following damage to several cortical and subcortical brain regions (Geschwind, 2009; Martinaud et al., 2009), though the frontal lobe, and specifically the prefrontal cortex, has been implicated most consistently and is the focus of this study. There remains a lingering tendency to refer to the wide array of personality and cognitive disturbances occurring with frontal lobe lesions as an undifferentiated “frontal lobe syndrome” (Carretero, Beamonte-Vela, Silvano-Cocinero, & Alvarez-Mendez, 2019). This is likely contributed to by the mélange of disturbances observed in conditions with widespread prefrontal dysfunction such as many traumatic brain injuries. However, the complex array of behavioral disturbances associated with frontal damage may be better understood with attention to distinct functional systems with distinct roles in personality that can be inferred from patterns of clinical-anatomical correlations (Burgess & Stuss, 2017; Eslinger & Damasio, 1985; Stuss & Benson, 1984). Accordingly, this study investigates patient with stable focal lesions, regardless of specific etiology. Challenges to this endeavor have included lack of standardized high-quality neuroimaging, lack of a reliable and valid instruments designed to measure acquired personality disturbances and insufficient numbers of suitable cases with focal lesions to draw reliable inferences (Stuss et al., 1992).
There are several validated instruments for assessment of personality in healthy or various clinical populations, but there is a paucity of assessments designed specifically for acquired personality disturbances (see Supplementary Material for further consideration of other approaches to personality assessment). This motivated the development and validation of the Iowa Scales of Personality Change (ISPC) (Barrash, Anderson, Hathaway-Nepple, Jones, & Tranel, 1997). The ISPC provides reliable and sensitive measurement of personality changes that occur in the setting of focal and non-focal brain injuries spanning multiple etiologies (Barrash, 2018). The 30-item scale has been characterized along four dimensions of disturbance using factor analysis (Barrash et al., 2011), including: (i) emotional and social personality disturbances (irascibility, emotional hyper-reactivity, interpersonal insensitivity and socially inappropriate behavior), (ii) dysexecutive personality disturbance (repeated real-life problems with planning, persistence and perseverative behavior), (iii) hypoemotional personality disturbance (emotional blunting and diminished drive), and (iv) distressed personality disturbance (enduring problems with anxiety, being easily overwhelmed and negative thinking). Recent analyses of ISPC data suggested that these personality disturbances are best evaluated as dimensional constructs, rather than categorical, and that a single type of disturbance was infrequent; co-occurrence of two or more disturbances at varying levels of severity was more common (Barrash et al., 2018). There are no studies directly investigating the correspondence of ISPC ratings and other instruments of personality assessment.
1.1. Study aims
The aim of this study was to extend investigation of heterogeneity in personality changes associated with frontal lobe damage by examining the neuroanatomical correlates of personality disturbances in patients with stable focal lesions due to varied etiologies. Theoretical premises for the study include (a) heterogeneity in personality disturbances reflects underlying dimensions of disturbance that often overlap, (b) these dimensions are partially instantiated in neural systems that are associated with different prefrontal cortex (PFC) sectors, and (c) these systems are integrated, so damage in one area of PFC cortex may disturb different types of personality disturbance to varying degrees. We use an observational, cross-sectional design, in a large sample of 182 individuals with well-characterized focal brain lesions and ISPC ratings of personality changes completed by family members. Hypotheses were informed by models of prefrontal functional-neuroanatomical systems presented by Cummings (Cummings, 1995) and Stuss (Stuss, 2011). Specifically, we hypothesized that: (a) emotional/social disturbance is associated with ventromedial PFC lesions; (b) hypoemotional disturbance is associated with dorsomedial PFC lesions; (c) dysexecutive personality disturbance is associated with dorsolateral PFC lesions; (d) distressed personality disturbance is not associated with focal PFC lesions in any region or in PFC in general. Two distinct analytic approaches were employed to investigate the brain-behavior associations: (i) hypothesis-driven stepwise regression analyses were employed to examine the effects of lesions in specific prefrontal regions of interest (ROI) on personality disturbances. (ii) data-driven multivariate lesion-symptom mapping, which identifies statistical associations between personality disturbances and the location of brain lesions. Lesion-symptom mapping was performed with the same anatomical hypotheses as presented above, but the analyses were not limited to a priori ROIs. Hypotheses, regions of interest (ROIs), and analytic methods were pre-registered at https://osf.io/tb43c. All changes to the pre-registered procedures and analysis plans are transparently identified, and the outcomes of pre-registered and post hoc analyses are distinguished in the Results.
2. Materials and methods
2.1. Participants
Participants included 182 individuals meeting study criteria, selected from the Patient Registry of the Division of Neuropsychology and Cognitive Neuroscience at the University of Iowa Department of Neurology. We report how we determined our sample size, all data exclusions (if any), all inclusion/exclusion criteria, all manipulations and all measures in the study. We confirm that all inclusion/exclusion criteria were established prior to data analysis. Inclusion criteria for the Registry include a single stable focal brain lesion with parenchymal damage evident on structural imaging, and exclusion criteria include a history of significant alcohol or substance abuse, psychiatric disorder, or other neurologic disorder unrelated to the lesion. Eligibility for the present study additionally required (a) the lesion was acquired at age 18 or older, (b) availability of high-quality structural neuroimaging data from the chronic epoch (at least three months after lesion onset), and (c) availability of valid ISPC ratings by an informant (spouse, parent, or adult child) completed at least four months after lesion onset. The last criterion is based on a judgment that this interval provides optimal balance between the competing considerations of (a) factors potentially compromising the validity of ratings, and (b) maximizing sample size (elaboration regarding this judgment is provided in Supplemental Material, section 2). Data collection for this study was continuous from 09/1997 to 4/2019 and all participants meeting the inclusion/exclusion criteria were included in the study, so the final sample size was determined by the cut-off of data collection. Etiologies causing the focal lesions included ischemic stroke, 62 (34.1%), surgical resection cavity following benign tumor resection, 40 (22.0%), hemorrhagic stroke, 38 (20.9%) — including 7 ruptured anterior communicating artery aneurysms, surgical resection for epilepsy, 30 (16.5%), traumatic brain injury with focal contusion, 6 (3.3%), herpes simplex encephalitis, 4 (2.2%), and anoxia, 2 (1.1%).
2.2. Procedures
All participants provided informed consent in accordance with federal and institutional guidelines, and all procedures were approved by the University of Iowa Institutional Review Board and are in accordance with the Declaration of Helsinki. All anatomical and personality data analyzed in this study were collected in the chronic epoch. Each participant also underwent neuropsychological testing according to standard procedures of the Benton Neuropsychology Laboratory (Tranel, 2009). Personality ratings were completed by an informant while the participant was engaged in cognitive testing.
2.3. Measures
2.3.1. Neuropsychological tests
For the purposes of this study, we included the Wechsler Adult Intelligence Scales to estimate general cognitive ability, Wechsler General Memory and Auditory Verbal Learning Test-Delayed Recall to assess memory, Trailmaking Test-Trail B to assess executive functioning, and Beck Depression Inventory to assess mood. The relationship of ISPC ratings and the self-reported Minnesota Multiphasic Personality Inventory scores was evaluated for a subset of participants with data available from both assessments. Legal copyright restrictions prevent public archiving of the various neuropsychological assessments used in this study, which can be obtained from the copyright holders in the cited references.
2.3.2. ISPC Personality ratings
The ISPC (Barrash et al., 1997) provides standardized assessment of 30 characteristics that might change as a result of a neurological condition, with characteristics concerning mood, affect, drive, social/interpersonal behavior, adaptive functioning, and cognitive functions such as flexibility, judgment/decision-making, self-reflectiveness and insight. This instrument can be accessed from GitHub: https://github.com/barrashj/APD-NACs_study.git. Four of the 30 items are control scales of characteristics that are not expected to develop disturbance as a consequence of brain damage, so ratings indicating marked change on these scales contribute to identification of invalid ratings. Ratings were made by a spouse or family member who knew the participant well and had regular interactions with the participant in a variety of situations both before and subsequent to lesion onset. Two ratings are made for each characteristic: “Before,” describing a patient’s typical functioning over their adult life prior to lesion onset, and “Now,” describing their functioning over the past year (or over the months since the acute epoch if the postmorbid period is less than a year). Characteristics are rated along 7-point scales, with higher ratings reflecting increased disturbance. Points along the scale are accompanied by rating guidelines with multiple behavioral examples to enhance reliability (Schwarz, 1999). Interrater agreement for the ISPC was found to be high across all scales, ranging from 0.80 to 0.96, and ratings have been found to be sensitive to different profiles of personality changes in different clinical groups (Barrash, 2018). There were no missing ISPC data for those scales included in study analyses.
2.3.3. Subtype disturbance scores
The primary behavioral variables in this study were disturbance scores for each personality subtype. Several steps were involved in calculating these scores. First, for individual personality items we collapsed ratings indicating no disturbance (ratings of 0–3) into a single “normal” rating, creating a 5-point scale: 0 (“no disturbance”), 1 (“mild disturbance”), 2 (“moderate disturbance”), 3 (“moderately severe disturbance”), and 4 (“severe disturbance”). This was done because we were specifically interested in disturbances in personality associated with the lesion, and individual differences between average and exemplary functioning are considered “noise” in the examination of our hypotheses. Next, we calculated the mean disturbance rating for each personality dimension derived from the following individual ISPC items: (i) emotional/social personality disturbance: irritability, impatience, socially inappropriate behavior, insensitivity, and inflexibility; (ii) dysexecutive personality disturbance: lack of planning, lack of persistence, perseverative behavior, and lack of initiative; (iii) hypoemotional personality disturbance: (a) blunted affect, apathy, and social withdrawal, and (b) those symptoms were not attributable to depression, they developed in the absence of depression (see Supplementary material for elaboration); and (vi) distressed personality disturbance: anxiety, depression and easily overwhelmed. Next, to quantify acquired disturbances associated with brain damage, we controlled for confounding effects of premorbid personality by conducting regression analyses for each dimension, with initial entry of the ISPC “Before” ratings for that dimension’s component items. This generates residualized disturbance scores with variance due to premorbid personality statistical removed, resulting in z scores that provide a common metric for all disturbances scores. Additional information regarding the loadings of individual ISPC scales on the four dimensions are presented in Supplementary Table 1. The code for calculating subtype disturbance scores can be accessed from GitHub: https://github.com/barrashj/APD-NACs_study.git.
2.4. Neuroanatomical analysis
2.4.1. Lesion segmentation
Each participant included in the analysis had a focal brain lesion with visible boundaries evident from research-quality structural imaging from T1 and T2 sequences on MRI, or CT in 6 individuals with MRI contraindications. Anatomical segmentation of lesion borders was traced manually for each subject and brought to a common template space for statistical analyses. The MAP-3 method of lesion tracing involves the manual tracing of lesion borders on a template brain using the lesion depicted in an MRI or CT scan as a guide, and has been described previously (H. Damasio & Frank, 1992; Fiez, Damasio, & Grabowski, 2000). With improvements in automated methods for transforming brains to a common space, lesions traced after 2006 were manually traced on native T1-weighted scans with FSL (Smith et al., 2004) and then transformed to the 1mm MNI152 atlas using nonlinear registration and lesion masking techniques available in ANTs (Avants, Epstein, Grossman, & Gee, 2008). Because lesions negatively affect the accuracy of the transformation to MNI space, transformations were performed using enantiomorphic normalization, which replaces the lesion volume with the voxel intensities from its non-damaged homologue to more closely align the transform with its template. Bilateral lesions were transformed by applying a cost function mask to the lesion volume (Brett, Leff, Rorden, & Ashburner, 2001), which reduces the influence of voxels within the lesion volume on the transformation process. The spatial transforms were then applied to the brain and lesion mask with nearest neighbor interpolation. The anatomical accuracy of the lesion tracing was reviewed in native and MNI space and edited as needed by a neurologist (A.D.B.) blinded to personality data.
2.4.2. A priori specification of prefrontal sectors and quantification of lesion overlap
The ventromedial, dorsomedial and dorsolateral PFC sectors were delineated a priori by co-investigator Donald Stuss, grouping specific cortical regions of the Glasser atlas (Glasser et al., 2016) to approximate architectonic subdivisions (Petrides & Pandya, 1994; Stuss et al., 2002). The ROIs are shown in Fig. 1. The masks for the ROIs are available upon request (see section 2.7 regarding data availability for details). Detailed specification of the atlas regions corresponding to the ROIs is presented in Supplementary Table 2. Neuroanatomical variables were the proportion of the specified region affected by lesion (voxels impacted by the lesion divided by total voxels within the ROI). Many lesions extended into more than one PFC sector.
Figure 1. Prefrontal Regions of Interest.
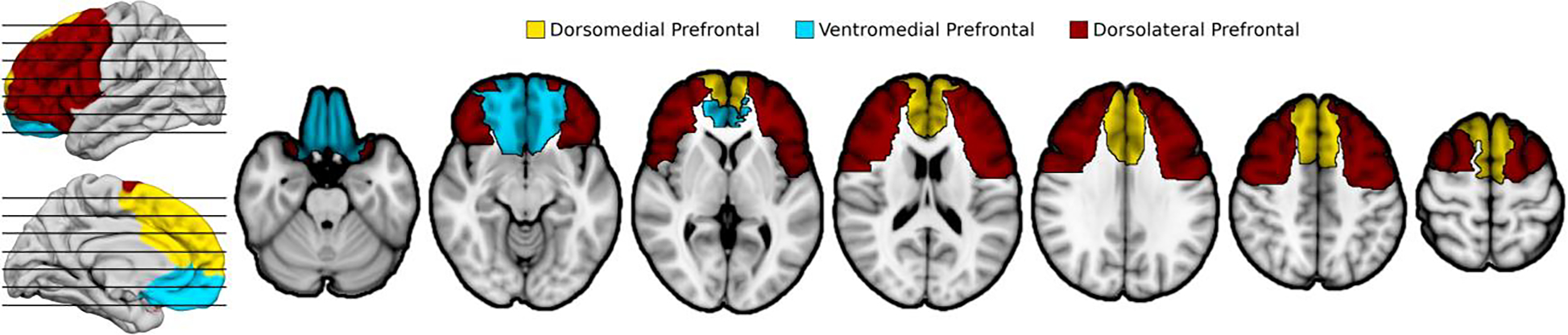
Prefrontal cortex was divided into three a priori ROIs prior to conducting analyses. The ventromedial PFC sector most likely includes cytoarchitectonic areas 12, 13, 14 and 25, and inferior aspects of 10, 11, 24 and 32. The dorsomedial PFC sector most likely includes medial aspects of 6, 8, 9 and 10, and superior aspects of 24 and 32. The dorsolateral PFC sector most likely includes 44, 45, 46, 47, lateral aspects of 6, 8 and 9, and superior aspects of 10 and 11. The ROIs required some manual modification to include underlying white matter, with pre-registration of the final ROIs.
2.5. Statistical analysis
All analyses involving behavioral data were performed with SPSS 27.0 (IBM, 2020), and code for these analyses can be accessed from GitHub: https://github.com/barrashj/APD-NACs_study.git. Potentially confounding variables were examined for associations with personality disturbances. These included gender (evaluated with t-tests), age, and interval between lesion acquisition and collection of personality data (evaluated with Pearson correlation coefficients), and relationship of rater to the patient (evaluated with ANOVA). Associations with overall lesion volume was examined and presented for descriptive purposes, but this variable is not statistically controlled for due to the premise of this study calling for examination of the associations of personality disturbance with ROIs, but parallel analyses controlling for lesion volume are reported in Supplementary material. Additionally, the association of cognitive scores to personality disturbances were also evaluated with Pearson correlations for informational purposes, but were not considered as potential confounds. Regarding the complex relationships of post-brain injury personality changes and cognitive deficits (especially executive dysfunction), previous in-depth analysis suggested that impairments seen on neuropsychological measures and corresponding personality changes were both related manifestations of frontal damage “with possible localizing value” (Tate, 1999). Accordingly, statistically controlling for associated cognitive deficits can be expected to introduce significant type II error to an investigation of the relationship between lesion location and personality changes in prefrontal patients. Finally, the relationship of ISPC ratings to measures of mood (assessed with BDI) and self-reported personality characteristics (MMPI-RF) were evaluated for descriptive purposes.
2.5.1. Regression analysis of ROIs and personality disturbances
Four parallel regression analyses with stepwise selection were employed to evaluate the hypotheses that damage to different PFC sectors was associated with the four personality disturbances. In the first step, the three PFC ROIs (ventromedial, dorsomedial and dorsolateral) each competed for selection into the regression equation at the requisite p ≤ 0.05 significance level. In the second step, the analysis examined whether any remaining ROIs could increase the variance in personality disturbance accounted for at the p ≤ 0.05 level, over and above variance accounted for by the ROI entering the equation in the first step. Regarding the distressed personality disturbance, it was hypothesized that this disturbance was not associated any specific PFC sector. Accordingly, in addition to the standard stepwise selection procedure, an additional regression analysis was performed with all three PFC ROIs entered as a block to evaluate whether this disturbance was associated with PFC damage in general. A departure from the analyses proposed in the OSF pre-registration relates to quantifying the extent of ROI lesioned as a continuous variable instead of the originally proposed binary categorization. This modification added further granularity to the ROI measures by incorporating the extent of injury and was suggested during the peer review process.
2.5.2. Follow-up examination of laterality effects
The possible effect of the laterality or bilaterality of a lesion on the association of a PFC region with a personality disturbance was examined in two steps. First, correlations were calculated to separately assess the relationship of damage in each of the nine neuroanatomical subregions (left, right and bilateral from each ROI) with personality disturbances. These were generally bivariate correlations, but a variable found to be a confound was to be included as covariate in partial correlations. These correlations may suggest patterns of associations, but they are limited by the fact that the neuroanatomical variables are not independent so the possible contribution of other ROIs to the correlations is unclear. To address this, in a second step, multivariate regression analysis with stepwise selection was employed to determine the subregion with the strongest unique relationship with each disturbance. After selection of the most highly related subregion, a second step determined whether another subregion could account for significant incremental variance in the disturbance.
2.6. Lesion-symptom mapping of acquired personality disturbances
Lesion-symptom mapping analyses were performed on the ISPC ratings using sparse canonical correlation analysis (SCCAN) as implemented in LESYMAP (Pustina, Avants, Faseyitan, Medaglia, & Coslett, 2018), a package available in R (https://github.com/dorianps/LESYMAP). The SCCAN method involves an optimization procedure that finds voxel weights that maximize the multivariate correlation between voxel values and disturbance scores, using residualized Z scores for each type of personality disturbance that was calculated as described above by covarying for premorbid personality ratings. The predictive value and sparseness of the model is derived empirically using a 4-fold, within-sample correlation between model-predicted and actual behavioral scores. LESYMAP deems a map “valid” if it is associated with a statistically significant predictive correlation. Briefly, SCCAN builds a model using 75% of the sample, applies it to the remaining 25% of the sample in order to predict the disturbance score in question from lesion location, and correlates these predictions with actual disturbance scores. Thus, this approach tests the predictive value of the entire map at once and avoids the pitfalls associated with voxel-wise (i.e., mass univariate) methods, such as inflated rates of false-positive errors. This previously validated method has been demonstrated to be more accurate than mass univariate methods and is better able to identify when multiple brain regions are associated with a behavioral variable (Pustina et al., 2018). Regions with minimal coverage (fewer than 3 lesions) were excluded to minimize the influence of regions with inadequate lesion coverage for the multivariate model, as performed previously (Bowren et al., 2020; Hindman et al., 2018).
2.7. Data availability
Anonymized study data are accessible from GitHub: https://github.com/barrashj/APD-NACs_study.git, with the exception of MRI data and lesion masks for which access is constrained by institutional policy requiring a signed data use agreement that is designed to ensure the appropriate use of the data for academic and not commercial purposes. The process by which investigators would acquire this data would be to email the corresponding author at joseph-barrash@uiowa.edu.
3. Results
3.1. Sample characteristics
The sample included 96 men (52.7%) and 86 women (47.3%) with a mean age of 53.3 ± 13.9 years (range, 20 to 85 years) and 13.8 ± 2.5 years of education. The age at onset of the brain lesion was 48.1 ± 14.4 years, with an interval of 5.2 ± 6.0 years (range, 4 – 360 months) between lesion acquisition and collection of personality data. Mean Verbal Comprehension Index from the Wechsler Adult Intelligence Scales was 101.5 ± 14.6, and the mean Perceptual-Organizational Index was 103.0 ± 14.2. The mean General Memory Index of the Wechsler Memory Scales was 100.2 ± 19.6. For reference, the population average for these scores is 100 ± 15. The mean score on the Beck Depression Inventory was 9.1 ± 8.0 (with mean score in the “minimal” range of depression). Analysis of the relationship of neuropsychological performances and personality disturbances is presented below in section 3.3.
3.2. Lesion distribution
The lesions were distributed throughout the brain with 91 of 182 involving the PFC. This included 46 ventromedial PFC lesions, 50 dorsomedial PFC lesions and 86 dorsolateral PFC lesions, with 56/91 PFC lesions involving more than one sector (as detailed in Supplementary Table 3). Another 91 lesions were distributed among posterior cortices as presented in Figure 2.
Figure 2. Lesion overlap maps.
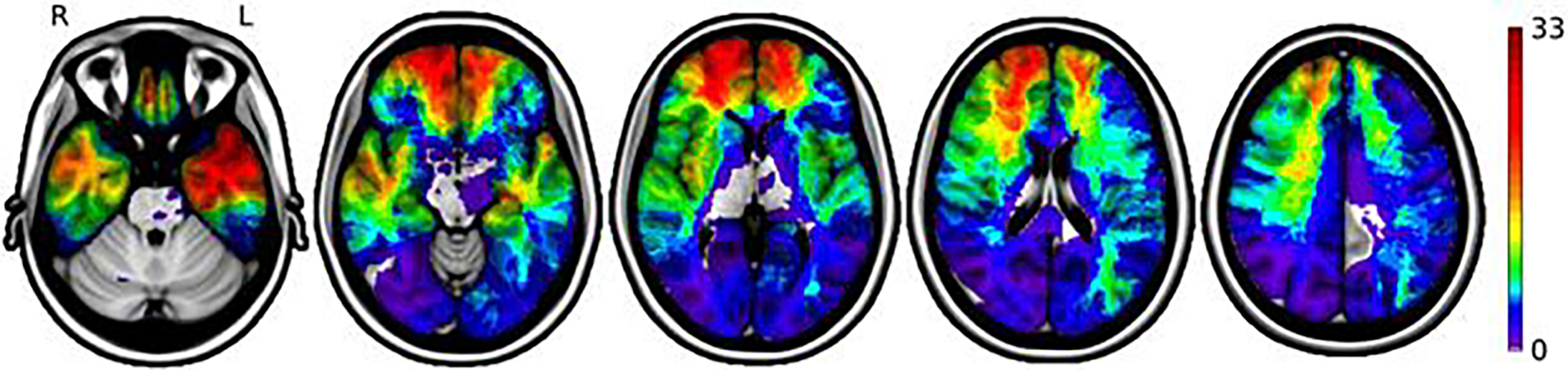
The distribution of brain lesions from 182 participants in the analysis is displayed on a color-coded scale, with greater lesion overlap shown in yellow and red colors. Regions with the highest coverage include the prefrontal cortex and temporal lobes bilaterally.
3.3. Relationship of ISPC ratings with other variables
Bivariate analyses of potentially confounding variables showed a significant effect of gender on emotional/social disturbance (t=2.84, p=.005). Men had a higher mean level of disturbance than women who, as a group, did not show disturbance (.19 and −.21, respectively). Accordingly, gender was controlled for in regression analyses of emotional/social disturbance. Lesion-symptom mapping for emotional/social disturbance was performed with and without controlling for gender. Gender effects did not approach significance (p > .22) for other personality disturbances. Age and interval between lesion onset and imaging, and between lesion onset and behavioral testing, were not significantly related to personality disturbances, nor was relationship of rater to the patient.
Correlations between neuropsychological measures and personality disturbances were also examined (presented in Supplementary Table 4). No neuropsychological scores were significantly correlated with emotional/social disturbance or hypoemotional disturbance. Several scores were correlated with dysexecutive disturbance including Full Scale IQ, Perceptual Organization Index, General Memory Index, Auditory Verbal Learning Test delayed recall, Trailmaking Test Trail B time (which was the most highly correlated score) and Beck Depression Inventory. Multivariate relationships of neuropsychological measures and personality disturbances was examined with multiple regression analyses (presented in Supplementary Table 5). These showed that once impairment on Trails B (a measure of executive functioning) is taken into account, no other cognitive variables accounted for significant variance in dysexecutive personality disturbance scores. The distressed personality disturbance was most highly correlated with Beck Depression Inventory score, and was also significantly correlated with Full Scale IQ, General Memory Index, and Auditory Verbal Learning Test delayed recall. Correlations of ISPC personality disturbances and self-reported MMPI scales were also calculated between higher-order scores from the ISPC and MMPI-RF (Supplementary Table 6), and between individual scales of the two measures (Supplementary Table 7). Both dysexecutive and emotional/social personality disturbances were correlated with behavioral/externalizing higher-order scales (r = 0.35 & 0.36, respectively) while the distressed ISPC scale was most highly correlated with the emotional/internalizing scale (r = 0.38).
3.4. Personality disturbance intercorrelations
Pearson correlations were significant between all pairs of disturbances (Table 1), with the strongest correlation between dysexecutive and distressed disturbances (r = .67) and weakest between emotional/social disturbance and hypoemotionality (r = .20). The severity of each acquired personality disturbance organized by lesion involvement in each PFC sector are presented in Supplementary Table 8.
Table 1.
Intercorrelations among personality disturbance scores
| Correlations between personality disturbances | |||
|---|---|---|---|
| Emotional/sociala | Dysexecutive | Hypoemotional | |
| Dysexecutive | .476*** | ||
| Hypoemotional | .199** | .520*** | |
| Distressed | .580*** | .674*** | .333*** |
Note.
Partial correlations between emotional/social disturbance and other disturbances, controlling for gender effect.
= <0.05
<0.01
< 0.001.
3.5. Regression analysis of PFC sectors and personality disturbances
Stepwise regression analyses of personality disturbances are presented in Table 2. For each disturbance, only one PFC sector was significantly associated with the disturbance, after which no other PFC sector contributed significant unique variance. Emotional/social disturbance, controlling for gender differences, was most strongly predicted by ventromedial PFC damage, with the model accounting for 8.7% of the variance in emotional/social disturbance (p < 0.001). Dysexecutive personality disturbance was most strongly predicted by ventromedial PFC damage, with the model accounting for 2.1% of dysexecutive variance (p = 0. 048). Hypoemotional disturbance was most strongly predicted by dorsomedial PFC damage, accounting for 2.3% of variance in hypoemotionality (p = 0.041). Distressed personality disturbance was not associated with any PFC sector, whether considered individually or collectively. Details regarding the relationships of lesion volume to extent of lesion in each ROI and to personality disturbances, and multivariate relationships of between ROI and personality disturbances controlling for lesion volume are presented in Supplementary Material, including Supplementary Table 9.
Table 2.
Stepwise regression analysis of the relationship of lesion location and personality disturbances
| Step | Variable | R2 | B | SE B | β | Sig. β | Sig. Model |
|---|---|---|---|---|---|---|---|
| Emotional-social personality disturbance | |||||||
| (Constant) | .519 | .223 | <.001 | ||||
| 1 | Gender | .041 | −.417 | −.210 | <−.210 | .004 | |
| 2 | Ventromedial damage | .087 | 1.192 | .215 | .215 | .003 | |
| Dysexecutive personality disturbance | |||||||
| (Constant) | −.065 | .080 | .048 | ||||
| 1 | Ventromedial damage | .021 | .813 | .409 | .146 | .048 | |
| Hypoemotional personality disturbance | |||||||
| (Constant) | .023 | −.066 | .080 | .41 | .041 | ||
| 1 | Dorsomedial damage | 1.125 | .548 | .151 | .041 | ||
| Distressed | |||||||
| No variables entered into the equation. | |||||||
Note. B = unstandardized regression coefficient; SE = standard error of B; β = standardized regression coefficient.
3.6. Examination of potential laterality effects
Stepwise regression analysis (Table 3) showed that emotional/social disturbance, controlling for gender, was most strongly predicted by left ventromedial lesions, with the model accounting for 13.1% of the variance in emotional/social disturbance (p < 0.001). No other subregion contributed significant incremental variance. Dysexecutive personality disturbance was most strongly predicted by bilateral dorsolateral lesions, with the model accounting for 5.2% of dysexecutive variance (p = 0.002); no other subregion contributed significant incremental variance. Hypoemotional personality disturbance was most strongly predicted by bilateral dorsolateral lesions, with the model accounting for 4.4% of hypoemotional variance (p = 0.004); no other subregion contributed significant incremental variance. No subregions were associated with distressed personality disturbance. The breakdown of PFC lesions by laterality are detailed in Supplementary Table 10, and correlations of these subregions to personality disturbance scores are presented in Supplementary Table 11.
Table 3.
Stepwise regression analysis to assess laterality effects
| Step entered | Variablea | R2 | B | SE B | β | Sig. β | Sig. Model |
|---|---|---|---|---|---|---|---|
| Emotional/social personality disturbance | |||||||
| (Constant) | .476 | .218 | <.001 | ||||
| 1 | Gender | .041 | −.396 | .139 | −.199 | .005 | |
| 2 | Left ventromedial damage | .131 | 3.082 | .717 | .299 | <.001 | |
| Dysexecutive personality disturbance | |||||||
| (Constant) | −.086 | .077 | .002 | ||||
| 2 | Bilateral dorsolateral damage | .052 | 3.591 | 1.138 | .229 | .002 | |
| Hypoemotional personality disturbance | |||||||
| (Constant) | .044 | −.080 | .078 | .004 | |||
| 1 | Bilateral dorsolateral damage | 3.302 | 1.143 | .210 | .004 | ||
| Distressed | |||||||
| No variables entered into the equation. | |||||||
Note. B = unstandardized regression coefficient; SE = standard error of B; β = standardized regression coefficient.
Nine neuroanatomical variables considered for entry into the regression equation for the four disturbances, each variable reflecting the proportion of a region that was damaged. The nine regions included: left, right and bilateral ventromedial PFC; left, right and bilateral dorsomedial PFC; and left, right and bilateral dorsolateral PFC.
3.7. Lesion-symptom mapping
The results of lesion-symptom mapping are shown in Figure 3. Emotional/social disturbance showed a peak finding in left ventromedial PFC white matter (r = 0.308; p = 2.28 ×10−5, peak MNI coordinate −23, 49, −2). Other significant peaks were present in white matter deep to the right dorsolateral PFC (25, 31, 22) and the right claustrum\insula (37, 0, 2). Lesion-symptom mapping was also performed for emotional/social disturbance with gender as a covariate. This analysis produced similar findings but more robust involvement of left ventromedial PFC, as shown in Supplementary Figure 1. Lesion-symptom mapping was also performed for men and women separately, and neither of these analyses produced significant results, likely reflecting inadequate power. Lesion-symptom mapping of dysexecutive personality disturbance demonstrated a significant peak finding within dorsolateral PFC, in right middle frontal gyrus (r = 0.163; p = 0.03, Fig. 3C, peak voxel 38, 49, 19). Lesion-symptom mapping of hypoemotional and distressed personality disturbances did not yield any significant findings.
Figure 3. Lesion-symptom maps.
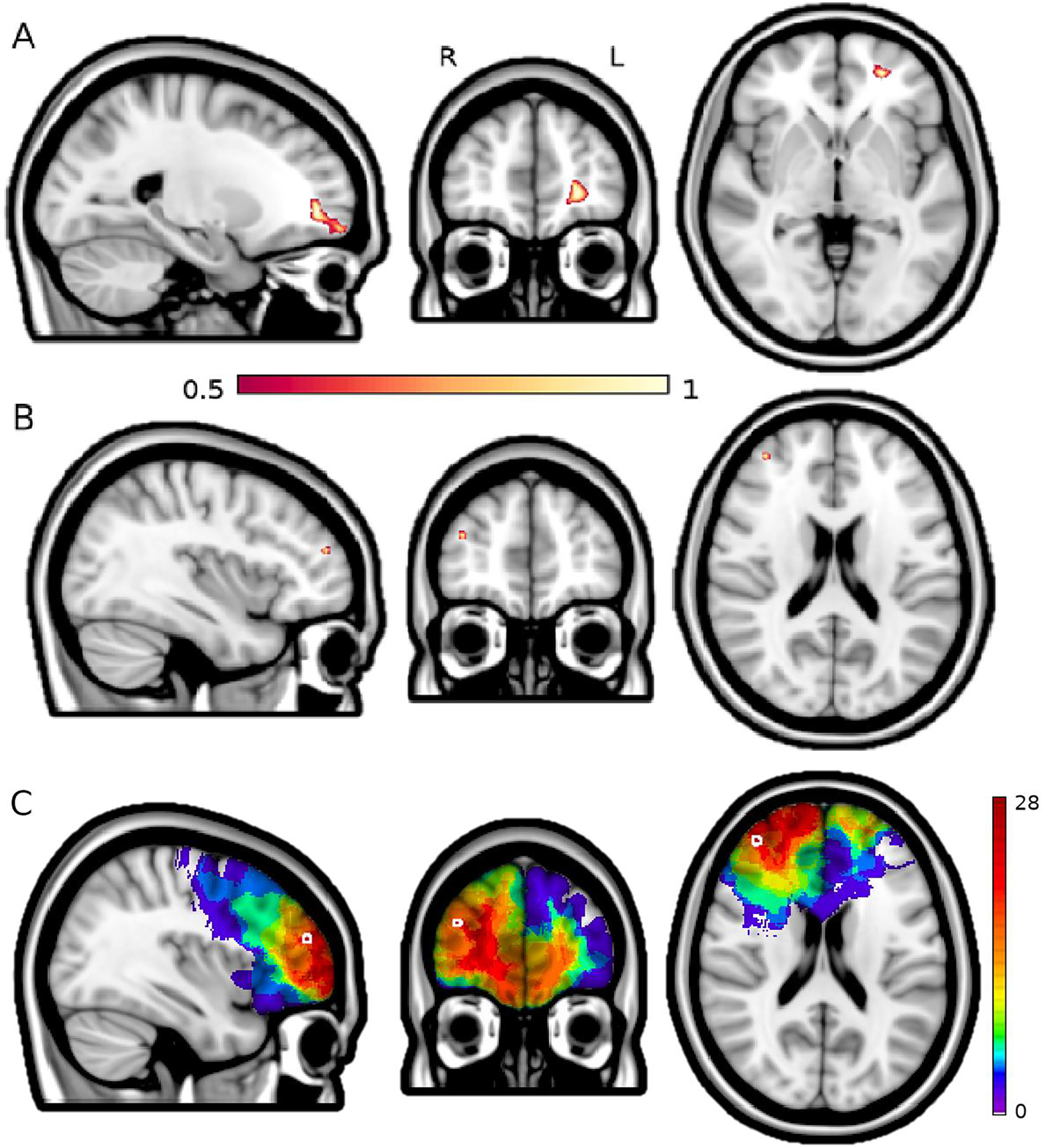
Panel A shows a region of the white matter deep to the left ventromedial PFC with the strongest association to emotional/social personality disturbance (p = 2.28 ×10−5, peak MNI coordinate −23, 49, −2). B shows a region in the right middle frontal gyrus of the dorsolateral prefrontal cortex that, when lesioned, is significantly associated with dysexecutive personality disturbance (p = 0.03, peak voxel 38, 49, 19). The color scale reflects the strength of association of anatomical regions with the respective personality disturbance score, with voxel weights distributed on a unit-less scale of 0–1 generated by the LESYMAP program to display the strength of regional associations within significant maps, which we thresholded at 0.5 to display the strongest findings within those maps. Panel C shows the distribution of lesions that intersect with the statistically significant region for dysexecutive personality disturbance (Panel B), thus contributing to that association.
4. Discussion
In this study we investigated hypotheses regarding the neuroanatomical correlates of acquired personality disturbances. Strengths of the study include the large sample with well-characterized, stable focal brain lesions, coupled with ratings of change in wide-ranging personality characteristics, made by family members with regular interactions with the participants before and after lesion onset, using an instrument validated for this purpose. Pre-registered hypotheses were evaluated with two distinct analytic approaches, one with personality disturbance as the dependent measure and the other with lesion location as dependent measure. Both approaches supported the hypothesized association between emotional/social personality disturbance and damage in ventromedial PFC, particularly on the left. Results were partially supportive of hypotheses concerning dysexecutive and hypoemotional personality disturbances. Dysexecutive personality disturbance was most strongly associated with damage in the ventromedial PFC in regression analysis, but when lesion laterality was taken into account it was most strongly associated with bilateral dorsolateral PFC lesions. Lesion-symptom mapping showed an area in the right dorsolateral PFC region to be maximally associated with dysexecutive disturbance. Hypoemotional disturbance was most strongly associated with damage in the dorsomedial PFC in regression analysis, as hypothesized. Lesion-symptom mapping did not yield any significant associations for hypoemotional disturbance. Although some statistically significant results were found in support of each hypothesis, it is noted that the magnitude of relationships between lesion location and personality disturbances was decidedly modest, and this tempers conclusions to be drawn from these results.
4.1. Acquired personality disturbances and neuroanatomical correlates
4.1.1. Emotional/social personality disturbance – ventromedial PFC
Emotional/social disturbance was most highly related to lesions in ventromedial PFC, especially the left ventromedial region. Those results were highly consistent with lesion-symptom mapping, which indicated maximal association of emotional/social disturbance with damage in the white matter of the ventromedial PFC extending to orbitofrontal cortex and frontal pole, likely involving Brodmann Area 11, the uncinate fasciculus and the inferior fronto-occipital fasciculus (Catani & de Schotten, 2012). In our sample, a gender effect was seen with men showing a significantly higher level of disturbance than women; when post hoc lesion-symptom mapping covaried for gender the results were again in the left ventromedial PFC. This region is part of the limbic network, as defined by resting state functional connectivity (Yeo et al., 2011). The association of emotional/social disturbance with ventromedial damage fits well with findings from increasingly sophisticated experimental paradigms in the cognitive and social neurosciences that have demonstrated an association of ventromedial PFC damage and emotional dysregulation or disturbed emotional experience, particularly in response to social stimuli (Anderson, Barrash, Bechara, & Tranel, 2006; Hornak, Rolls, & Wade, 1996; Jenkins et al., 2018; Moll et al., 2011). Irritability, impatience and lability are common manifestations (Barrash, Tranel, & Anderson, 2000; Hornak et al., 2003; Zald, Mattson, & Pardo, 2002) along with deficits in abilities critical to interpersonal sensitivity and socially appropriate behavior (Rowe, Bullock, Polkey, & Morris, 2001; Shamay-Tsoory, Aharon-Peretz, & Perry, 2009; Stuss, Gallup Jr, & Alexander, 2001), including deficient self-monitoring of social behavior (Beer, John, Scabini, & Knight, 2006). A compelling case has been made that disturbances of emotional experience impair the decision-making process because of the importance of emotional processing in the decision-making process, which includes moral reasoning and judgment (Bechara, Tranel, & Damasio, 2000; A. R. Damasio, 1994). Consistent with this work, striking emotional changes and social disturbances with ventromedial PFC lesions have been carefully documented in other case studies (M. P. Alexander & Freedman, 1984; Dimitrov, Phipps, Zahn, & Grafman, 1999; Eslinger & Damasio, 1985) and group studies (DeLuca & Diamond, 1995; Eslinger & Damasio, 1984; Grafman et al., 1996; Hornak et al., 2003; Logue et al., 1968; Rolls, Hornak, Wade, & McGrath, 1994; Sarazin et al., 1998; Steinman & Bigler, 1986; Storey, 1970). Such disturbances may be especially severe when the ventromedial PFC lesion onset is early in life (Anderson, Bechara, Damasio, Tranel, & Damasio, 1999; Boes et al., 2011).
There is evidence that acquired damage to the polar prefrontal region can in some cases be associated with improved functioning in the various personality characteristics measured by the ISPC (King, Manzel, Bruss, & Tranel, 2020). Notably, this association was strongest with right prefrontal lesions. A similar result was obtained for ratings of psychological well-being on scales measuring “eudaimonic well-being” including such attributes as self-acceptance, purpose in life, and personal growth (Ryff, 1989). While these findings require further investigation to understand potential mechanisms, it is possible that an association between right prefrontal damage and positive change (in a small subset of patients) could contribute to the weaker result in the current study for lesions in right ventromedial PFC and acquired personality disturbances, compared to left ventromedial PFC.
4.1.2. Dysexecutive personality disturbance – Dorsolateral PFC
Regression analysis of ROIs found dysexecutive personality disturbance was most highly associated with ventromedial lesions, rather than hypothesized dorsolateral lesions. However, when laterality was taken into account, bilateral dorsolateral lesions were the strongest predictor, explaining 5.2% of the variance in dysexecutive personality disturbance (compared to 2.1% associated with ventromedial lesions when laterality was not taken into account). Lesion-symptom mapping found that the peak region for lesions associated with dysexecutive personality disturbance was in the right middle frontal gyrus around the junction of Brodmann Areas 45 & 46. This region is within the fronto-parietal and salience\ventral attention B networks (Schaefer et al., 2018; Yeo et al., 2011) and has previously been associated with an array of executive cognitive processes (Burgess & Stuss, 2017; Fuster, 1997; Goldman-Rakic, 1992; Hwang, Bruss, Tranel, & Boes, 2020; Milner, Petrides, & Smith, 1985; Stuss, 2011). Results are consistent with studies directly contrasting the effects of dorsolateral PFC and ventromedial PFC lesions that find dissociations, with dorsolateral PFC lesions associated with impaired cognitive control and the latter associated with deficits in emotional/social behavior and decision-making (Bechara, Damasio, Tranel, & Anderson, 1998; Bechara et al., 2000; Beer et al., 2006; Gilbert et al., 2006; Gläscher et al., 2012; Robinson, Calamia, Gläscher, Bruss, & Tranel, 2014; Shamay-Tsoory et al., 2009).
4.1.3. Hypoemotional personality disturbance – dorsomedial PFC
The hypothesis that hypoemotional disturbance is associated with dorsomedial PFC damage was weakly supported. The primary analysis of ROIs indicated that lesions of the dorsomedial PFC were most associated with hypoemotional disturbance, but when laterality was taken into account, stepwise regression analysis showed that bilateral dorsolateral lesions were more highly associated with hypoemotional disturbance, accounting for 4.4% of variance. This unexpected finding is likely attributable to the high degree of overlap (67%) of bilateral dorsomedial lesions with co-occurring bilateral dorsolateral PFC damage (which typically occurred in the context of larger lesions involving two or three PFC sectors). Lesion-symptom mapping did not produce significant findings. This hypothesized dorsomedial region is associated with the initiation of motivated behavior (Husain & Roiser, 2018) and behavioral apathy (Bonnelle, Manohar, Behrens, & Husain, 2016), and prior case reports document that lesions of this region are associated with a syndrome including apathy, behavioral inertia, akinesia, mutism, and deficits in awareness of and reflection on emotional states (Barris & Schuman, 1953; Campanella, Shallice, Ius, Fabbro, & Skrap, 2014; Cohen et al., 1999; A. R. Damasio & Van Hoesen, 1983; Laplane, Degos, Baulac, & Gray, 1981; Nielsen & Jacobs, 1951; Schäfer et al., 2007; Wilson & Chang, 1974), and electrical stimulation of the anterior cingulate within this region is associated with the will to persevere (Parvizi, Rangarajan, Shirer, Desai, & Greicius, 2013). Initially dramatic impairments often improve significantly (A. R. Damasio & Van Hoesen, 1983), but disturbances may persist (Cohen et al., 1999; Hornak et al., 2003). The effects of damage to this region contrast with impaired decisions with ventromedial PFC damage (Rushworth, Behrens, Rudebeck, & Walton, 2007).
4.1.4. Distressed personality disturbance
As hypothesized, distressed personality disturbance was not associated with lesions in any PFC sectors in any regression analysis or in lesion-symptom mapping. It has been found previously that distressed personality disturbance was not associated with ventromedial PFC damage (Barrash et al., 2011), and this study extends this finding to other PFC sectors and to regions outside of the PFC. This personality disturbance’s lack of association with PFC and with non-PFC lesions speaks to the specificity of findings for other personality disturbances. That is, not all personality disturbances following brain damage are attributable to PFC damage.
4.1.5. Implications of neuroanatomical correlates for PFC systems
Taken together, these results suggest differing roles for PFC sectors in personality. The findings are largely consistent with a models of prefrontal functioning elaborated by Stuss and colleagues (Stuss, 1992, 2011; Stuss & Alexander, 2007; Stuss et al., 2002; Stuss & Benson, 1984) and by Cummings (Cummings, 1993, 1995). These models emphasize distinctive roles for different areas grounded in early cytoarchitectural and myeloarchitectural investigation of human brain development. This includes ventromedially located affective circuitry evolving from olfactory cortices, with rich bidirectional connections with the subgenual anterior cingulate cortex and posterior limbic regions via the uncinate fasciculus. A second system includes dorsally located ‘cognitive’ circuitry evolved from hippocampal cortices, with rich connections to virtually all posterior association cortices, as well as ventral and medial prefrontal sectors (G. E. Alexander, DeLong, & Strick, 1986; A. R. Damasio & Anderson, 2003; Pandya & Barnes, 1987; Pandya & Yeterian, 1996; Sanides, 1964; Wise, 2008). A third dorsomedial system developed from the dorsal anterior cingulate cortex that is heavily interconnected and all aspects of the limbic system, via the cingulum and other white matter pathways (G. E. Alexander et al., 1986). It has been observed that “understanding the prefrontal lobe depends upon knowledge of the company it keeps, its afferent and efferent connections” (A. R. Damasio & Anderson, 2003). The unique connectional patterns of these circuits are consistent with neuroanatomically-segregated PFC systems developed for control over different aspects of behavior (Stuss, 1992). Specifically, the rich connections between the ventromedial region and the limbic system permit for control of emotional reactions and behavioral inhibition; the largely bidirectional connections of dorsolateral cortex with posterior cortices enables executive control over cognition and behavior; and the connections of the dorsomedial PFC with the dorsal anterior cingulate and other limbic structures infuse motivation and energization to the “affective” and the “cognitive” systems.
Differential patterns of neuroanatomical and neuropsychological changes between systems further support their basic distinctiveness (Phillips, MacPherson, & Della Sala, 2002). Neuroscientific research, briefly reviewed above, has illuminated an impressive array of highly detailed aspects of systems that have apparently evolved for higher-level cortical control over emotional regulation and social behavior, executive control of cognition and behavior, and drive and activation, and the neuroscientific literature has informed and supported the models of functionally distinct prefrontal systems. The present study suggests that damage to circuitry of prefrontal systems may result in observable, enduring, cross-situational disturbance in real-life functioning, with the nature of the disturbance related to impairments in the basic role of the involved system.
The pattern of findings also demonstrates the value of differentiating between types of acquired personality disturbances, in contrast to studies that have investigated the neuroanatomical correlates of personality disturbances that were analyzed collectively as a multifaceted set of disturbances in “control functions” (Godefroy, 2003; Godefroy et al., 2010); that is, with the grouping together disparate aspects of the four types of acquired personality disturbances investigated in this study. With specific types of personality disturbance not taken into account, the heterogeneous set of personality disturbances failed to show an association with damage in any cortical region (although an association was seen with left ventral striatum damage) (Martinaud et al., 2009).
4.2. Implications regarding personality broadly
The current findings fit well within the framework of existing models of PFC contributions to adaptive behavior (Cummings, 1993; Stuss et al., 2002) and how focal brain lesions may disrupt adaptive behavior. Beyond this, the findings have implications for our understanding of the neuropsychological basis of personality and personality disorders. Personality is generally appreciated as one of the most important factors in individual identity, quality of life and social success. However, it is also necessarily a somewhat vague concept with blurred boundaries. As an obviously multifaceted construct, the component structure and dynamics of personality have been topics of interest since long before the advent of modern neuroscience. The current study brings to this discussion the analysis of a large sample of focal PFC lesions linked to detailed assessment of personality disturbances grouped into empirically-derived higher-order dimensions. Employment of the lesion method permits conclusions that are difficult to arrive at by other imaging or clinical methods: when a given function is disrupted by a focal lesion, it implies that the damaged brain region is at least partly necessary for that function (H. Damasio & Damasio, 1989). Each of the three PFC sectors studied here were associated with disruption of aspects of personality, albeit at varying levels of robustness. These components, broadly defined, include emotional/social functioning, drive and activation, and executive control of real-life behavior, and they appear to be required for personality to operate adaptively. Accordingly, they are necessary components of any neuroscientifically-sound theory of normal personality function and personality disorders. There is no implication that personality is limited to the interaction of these three components, only that they are critical components. Integration of dissociable PFC systems with non-frontal brain regions clearly is essential for normal personality function (Mulders, Llera, Tendolkar, van Eijndhoven, & Beckmann, 2018; Simon, Varangis, & Stern, 2020), especially limbic regions involved in emotion (Adolphs, 2009). At a simplistic level, personality may be conceptualized as involving a dynamic interaction among these three major aspects of personality (Allemand, Zimprich, & Hertzog, 2007), in concert with emotional and cognitive processes, with each system functioning somewhere along a continuum from optimal function to severe dysfunction. Selective dysfunction of any component could be caused by factors less blatant than the focal lesions studied here, e.g., developmental neural migratory disorders (Boes et al., 2011). The relative “strength” of one system compared to the others, determined by some combination of genetic and experiential factors, could contribute to different personality tendencies (Mahoney, Rohrer, Omar, Rossor, & Warren, 2011). That said, personality clearly is more complex than the interaction of three systems. The modest variance explained by focal lesions to these PFC sectors observed here attests to this. It is likely that each of the three PFC systems can be further subdivided in concert with more nuanced functional roles, and with contributions from non-frontal limbic structures and posterior association cortices. This is fertile ground for future study.
4.3. Limitations
This study has several limitations. Foremost is the topographical distribution of lesions, with lesions often involving more than one PFC sector, a limitation that was particularly evident with bilateral dorsolateral lesions, which typically also involved dorsomedial and ventromedial regions. This limitation is inherent to naturally-occurring brain lesions, but it reduces the clarity of each sector’s specific contribution to personality disturbances. Additionally, although coverage was generally strong for the PFC ROIs in this study, several non-PFC regions and some aspects of PFC sectors did not have sufficient lesion coverage in the multivariate lesion-symptom mapping analyses, so these analyses do not provide a basis for inferences regarding contributions from areas throughout the brain. There is evidence that subcortical lesions in frontal-subcortical circuits can cause the same behavioral syndromes as cortical lesions (Cummings, 1993), and specific loci in subcortical structures appear to be associated with personality disturbances (Corbetta et al., 2015; De Simoni et al., 2018; Hoffmann, 2013; Koziol & Budding, 2009; Strub, 1989). We lacked lesion coverage in many of these subcortical regions. Relatedly, analyses were limited to three broad PFC sectors with the frontal pole included primarily within ventromedial PFC. The set of lesions in our data set did not allow for separate analysis of possible effects of polar damage, specifically, due to the co-occurrence of damage in ventromedial cortex more broadly. Nevertheless, there is some evidence that the frontal pole may serve higher order integration of emotional processing, motivation, energization, and executive capacities (Stuss, 2011; Stuss & Alexander, 2007; Stuss et al., 2002), and this would be consistent with the observed association in our sample of dysexecutive disturbance associated with ventromedial lesions. Follow-up analyses indicate that laterality effects may be important, and prior research has indicated that laterality effects of ventromedial lesions may vary according to gender (Tranel, Damasio, Denburg, & Bechara, 2005). Another methodological issue concerns etiology as there are advantages to studies that are limited to a specific etiology. For the neuroanatomical hypotheses we set out to test, our study design called for inclusion of diverse etiologies. A primary advantage of this design was to accumulate the largest possible cohort of individuals with acquired focal brain lesions, and additional advantages come by way of a more diverse topography of lesions, and the potential for a greater diversity of patients as age and risk factors often differ with different etiologies.
On the behavioral side of the analysis, it is emphasized that many patients have a mixture of two or more types of disturbance, which has been observed previously (Stout, Ready, Grace, Malloy, & Paulsen, 2003; Stuss & Benson, 1984), suggesting that acquired disturbances in the different types of personality dimensions are not independent events and that lesions may disrupt several aspects of personality functioning. Additionally, that naturally-occurring lesions in the present study most often involve multiple PFC sectors likely contributed to substantial overlap among personality disturbances. Another potential limitation concerns raters who are not trained professionals. The relationship of the rater to the patient was not significantly related to personality disturbances; however, we note that we do not have the relevant data on raters and it is possible that characteristics of the raters (e.g., rater’s mood, the quality of their relationship with the patient, or rater’s education) may have influenced their ratings of the patient (McKinlay & Brooks, 1984; Tate, 1999). If so, however, such factors would add noise to ratings, resulting in observed variances accounted for by lesion location that underestimate the true effect of lesion location. Finally, the relatively few core characteristics employed in this study to define personality disturbances (based on earlier factor analysis) may not provide as complete a characterization of the disturbances as would be clinically useful.
4.4. Clinical relevance & future directions
Personality disturbances after brain injury may result in greater psychosocial disability than would be expected by cognitive status (Barrash et al., 2020; Lezak, 1989). Increased understanding of the roles of different sectors of the PFC in emotional, cognitive and behavioral processes may improve our ability in the clinical setting to identify or even anticipate syndromes in patients with focal PFC lesions, and this has the potential to enhance development of targeted rehabilitation approaches to ameliorate the consequences of personality disturbances (Arnemann et al., 2015; Cicerone, Levin, Malec, Stuss, & Whyte, 2006; D’Esposito & Chen, 2006; Hoffmann, 2013; Lane-Brown & Tate, 2009; Levine et al., 2011; Santangelo et al., 2018). The hypothesized association between specific personality disturbances and damage to distinct PFC sectors was based on decades of accumulated knowledge through case reports and group analyses, as referenced above. The fact that only a small amount of the variance in personality (<10%) could be explained by the anatomical location of the damage could be viewed as a call to action to revise and improve upon these complex brain-behavior relationships. Future studies may further refine the relationship of personality disturbance and lesion location with a much larger sample size and judicious inclusion/exclusion criteria for specific anatomical features may permit further delineation of PFC regions, possibly revealing stronger associations with personality disturbances than those seen with very broad PFC sectors. A larger sample size and fine-grained analyses may also permit a more complete characterization of the specific aspects of personality changes that comprise different personality disturbances. Important future directions also include leveraging a growing database for discerning possible effects of damage to the frontal pole specifically, and to specific subcortical structures to the extent possible, as well as further investigation of laterality and gender effects.
In conclusion, this study features a large sample with well-characterized, stable, focal lesions in different PFC and non-PFC areas, and detailed ratings of personality changes by family who regularly observe patients’ behavior across a wide range of real-life circumstances. Though discrete dissociations were not observed, results were generally consistent with hypothesized patterns of association between lesions in with different PFC sectors and different personality disturbances, and the pattern of results tended to be consistent across both region-of-interest (anatomy-to-behavior) and data-driven (behavior-to-anatomy) analytic approaches. This study constitutes an important step in our understanding of control/dyscontrol of real-life behavior by functionally-related systems distributed in PFC sectors, and this may contribute to more accurate prognostic information for patients and their families regarding personality changes that may occur following a brain lesion, along with aiding the development of tailored rehabilitation strategies. The results highlight that the term “frontal lobe syndrome” is anachronistic and has outlived whatever usefulness it may have had, and communication in clinical and neuroscientific endeavors will benefit from terminology that more precisely conveys the type of disturbance(s) present.
Supplementary Material
Highlights.
Emotional/social disturbance was associated with ventromedial prefrontal lesions
Dysexecutive disturbance was associated with bilateral dorsolateral lesions
Hypoemotional disturbance was associated with dorsomedial prefrontal lesions
Distressed personality disturbance was not specifically associated with focal prefrontal lesions
There is much overlap in personality disturbances caused by focal prefrontal lesions
Acknowledgements
The authors thank the participants and their families for their contributions to this study, and Nazan Aksan, Ph.D. and Mark Bowren, M.A. for statistical consultation and analysis at early stages of the study, and reviewers for their insightful suggestions. We are especially grateful to Professor Donald Stuss (9/26/1941 – 9/3/2019) for his integral involvement in this study’s theoretical underpinnings and earlier work. His important insights on frontal lobe function and its role in personality over the course of his career and in the current project have been extraordinary. His many contributions serve as an inspiration to us.
Funding
This research was partially supported by grants from the National Institutes of Mental Health (P50 MH094258 to DT; R01NS114405 to ADB; and R21MH120441 to ADB), and the Kiwanis Neuroscience Research Foundation (to DT). This work was conducted with an MRI instrument funded by 1S10OD025025–01. The funding sources had no involvement in the design or execution of this study.
Abbreviations:
- ISPC
Iowa Scales of Personality Change
- PFC
prefrontal cortex
- ROI
region of interest
Footnotes
Competing Interests
The authors have no competing interests to declare.
Supplementary material
Supplementary material will be available online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R (2009). The social brain: neural basis of social knowledge. Annual review of psychology, 60, 693–716. doi: 10.1146/annurev.psych.60.110707.163514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, & Strick PL (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience, 9(1), 357–381. doi: 10.1146/annurev.ne.09.030186.002041 [DOI] [PubMed] [Google Scholar]
- Alexander MP, & Freedman M (1984). Amnesia after anterior communicating artery aneurysm rupture. Neurology, 34(6), 752–752. doi: 10.1212/wnl.34.6.752 [DOI] [PubMed] [Google Scholar]
- Allemand M, Zimprich D, & Hertzog C (2007). Cross- sectional age differences and longitudinal age changes of personality in middle adulthood and old age. Journal of personality, 75(2), 323–358. doi: 10.1111/j.1467-6494.2006.00441.x [DOI] [PubMed] [Google Scholar]
- Anderson SW, Barrash J, Bechara A, & Tranel D (2006). Impairments of emotion and real-world complex behavior following childhood-or adult-onset damage to ventromedial prefrontal cortex. Journal of the International Neuropsychological Society, 12, 224–235. doi:10.10170S1355617706060346 [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, & Damasio AR (1999). Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature neuroscience, 2(11), 1032–1037. doi: 10.1038/12194 [DOI] [PubMed] [Google Scholar]
- Arnemann KL, Chen AJW, Novakovic-Agopian T, Gratton C, Nomura EM, & D’Esposito M (2015). Functional brain network modularity predicts response to cognitive training after brain injury. Neurology, 84(15), 1568–1574. doi: 10.1212/wnl.0000000000001467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, & Gee JC (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical image analysis, 12(1), 26–41. doi: 10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J (2018). Iowa Scales of Personality Change. In Kreutzer JS, DeLuca J, & Caplan B (Eds.), Encyclopedia of clinical neuropsychology (2nd ed., pp. 74–76). New York: Springer. [Google Scholar]
- Barrash J, Abel TJ, Okerstrom-Jezewski KL, Zanaty M, Bruss JE, Manzel K, . . . Tranel D (2020). Acquired personality disturbances after meningioma resection are strongly associated with impaired quality of life. Neurosurgery, 87, 276–284. doi: 10.1093/neuros/nyz440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Anderson SW, Hathaway-Nepple J, Jones RD, & Tranel D (1997). Iowa Scales of Personality Change. Iowa City, IA: University of Iowa, Department of Neurology. [Google Scholar]
- Barrash J, Asp E, Markon K, Manzel K, Anderson SW, & Tranel D (2011). Dimensions of personality disturbance after focal brain damage: investigation with the Iowa Scales of Personality Change. J Clin Exp Neuropsychol, 33(8), 833–852. doi: 10.1080/13803395.2011.561300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Stuss DT, Aksan N, Anderson SW, Jones RD, Manzel K, & Tranel D (2018). “Frontal lobe syndrome”? Subtypes of acquired personality disturbances in patients with focal brain damage. Cortex, 106, 65–80. doi: 10.1016/j.cortex.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Tranel D, & Anderson SW (2000). Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol, 18(3), 355–381. doi: 10.1207/S1532694205Barrash [DOI] [PubMed] [Google Scholar]
- Barris RW, & Schuman HR (1953). Bilateral anterior cingulate gyrus lesions. Syndrome of the anterior cingulate gyri. Neurology. doi: 10.1212/WNL.3.1.44 [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, & Anderson SW (1998). Dissociation of working memory from decision making within the human prefrontal cortex. Journal of Neuroscience, 18(1), 428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, & Damasio H (2000). Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain, 123(11), 2189–2202. doi: 10.1093/brain/123.11.2189 [DOI] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D, & Knight RT (2006). Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. Journal of cognitive neuroscience, 18(6), 871–879. doi: 10.1162/jocn.2006.18.6.871 [DOI] [PubMed] [Google Scholar]
- Boes AD, Grafft AH, Joshi C, Chuang NA, Nopoulos P, & Anderson SW (2011). Behavioral effects of congenital ventromedial prefrontal cortex malformation. BMC neurology, 11(1), 151. doi: 10.1186/1471-2377-11-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Manohar S, Behrens T, & Husain M (2016). Individual differences in premotor brain systems underlie behavioral apathy. Cerebral Cortex, 26(2), 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowren M, Adolphs R, Bruss J, Manzel K, Corbetta M, Tranel D, & Boes AD (2020). Multivariate lesion-behavior mapping of general cognitive ability and its psychometric constituents. Journal of Neuroscience, 40(46), 8924–8937. doi: 10.1523/JNEUROSCI.1415-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, & Ashburner J (2001). Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage, 14(2), 486–500. doi: 10.1006/nimg.2001.0845 [DOI] [PubMed] [Google Scholar]
- Burgess PW, & Stuss DT (2017). Fifty years of prefrontal cortex research: Impact on assessment. Journal of the International Neuropsychological Society, 23(9–10), 755–767. doi: 10.1017/S1355617717000704 [DOI] [PubMed] [Google Scholar]
- Campanella F, Shallice T, Ius T, Fabbro F, & Skrap M (2014). Impact of brain tumour location on emotion and personality: a voxel-based lesion–symptom mapping study on mentalization processes. Brain, 137(9), 2532–2545. doi: 10.1093/brain/awu183 [DOI] [PubMed] [Google Scholar]
- Carretero RG, Beamonte-Vela B-N, Silvano-Cocinero J-D, & Alvarez-Mendez A (2019). Behavioural changes as the first manifestation of a silent frontal lobe stroke. BMJ Case Reports CP, 12(1). doi: 10.1136/bcr-2018-227617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, & de Schotten MT (2012). Atlas of human brain connections. Oxford, UK: Oxford University Press. [Google Scholar]
- Cicerone K, Levin H, Malec J, Stuss D, & Whyte J (2006). Cognitive rehabilitation interventions for executive function: moving from bench to bedside in patients with traumatic brain injury. Journal of cognitive neuroscience, 18(7), 1212–1222. doi: 10.1162/jocn.2006.18.7.1212 [DOI] [PubMed] [Google Scholar]
- Cohen RA, Kaplan RF, Zuffante P, Moser DJ, Jenkins MA, Salloway S, & Wilkinson H (1999). Alteration of intention and self-initiated action associated with bilateral anterior cingulotomy. The Journal of neuropsychiatry and clinical neurosciences, 11(4), 444–453. doi: 10.1176/jnp.11.4.444 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS, . . . Lang CE (2015). Common behavioral clusters and subcortical anatomy in stroke. Neuron, 85(5), 927–941. doi: 10.1016/j.neuron.2015.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL (1993). Frontal-subcortical circuits and human behavior. Archives of Neurology, 50(8), 873–880. doi: 10.1001/archneur.1993.00540080076020 [DOI] [PubMed] [Google Scholar]
- Cummings JL (1995). Anatomic and behavioral aspects of frontal- subcortical circuits. Annals of the New York Academy of Sciences, 769(1), 1–14. doi: 10.1111/j.1749-6632.1995.tb38127.x [DOI] [PubMed] [Google Scholar]
- D’Esposito M, & Chen AJW (2006). Neural mechanisms of prefrontal cortical function: implications for cognitive rehabilitation. Progress in brain research, 157, 123–392. doi: 10.1016/S0079-6123(06)57008-6 [DOI] [PubMed] [Google Scholar]
- Damasio AR (1994). Descartes’ error: Emotion, reason, and the human brain. New York: Grosset and Putnam. [Google Scholar]
- Damasio AR, & Anderson SW (2003). The frontal lobes. In Heilman K & Valenstein E (Eds.), Clinical neuropsychology (4th ed., pp. 404–446). New York: Oxford University Press. [Google Scholar]
- Damasio AR, & Van Hoesen GW (1983). Emotional disturbances associated with focal lesions of the limbic frontal lobe. In Heilman KM & Satz P (Eds.), Neuropsychology of Human Emotion (pp. 85–110). New York: Guilford. [Google Scholar]
- Damasio H, & Damasio AR (1989). Lesion analysis in neuropsychology. In. New York: Oxford University Press. [Google Scholar]
- Damasio H, & Frank R (1992). Three-dimensional in vivo mapping of brain lesions in humans. Arch Neurol, 49(2), 137–143. doi: 10.1001/archneur.1992.00530260037016 [DOI] [PubMed] [Google Scholar]
- De Simoni S, Jenkins PO, Bourke NJ, Fleminger JJ, Hellyer PJ, Jolly AE, . . . Sharp DJ (2018). Altered caudate connectivity is associated with executive dysfunction after traumatic brain injury. Brain, 141(1), 148–164. doi: 10.1093/brain/awx309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca J, & Diamond BJ (1995). Aneurysm of the anterior communicating artery: A review of neuroanatomical and neuropsychological sequelae. J Clin Exp Neuropsychol, 17(1), 100–121. doi: 10.1080/13803399508406586 [DOI] [PubMed] [Google Scholar]
- Dimitrov M, Phipps M, Zahn TP, & Grafman J (1999). A thoroughly modern Gage. Neurocase, 5(4), 345–354. doi: 10.1080/13554799908411987 [DOI] [Google Scholar]
- Eslinger P, & Damasio AR (1984). Behavioral disturbances associated with rupture of anterior communicating artery aneurysms. Seminars in Neurology, 4(3), 385–389. doi: 10.1055/s-2008-1041568 [DOI] [Google Scholar]
- Eslinger P, & Damasio AR (1985). Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology, 35(12), 1731–1741. doi: 10.1212/WNL.35.12.1731 [DOI] [PubMed] [Google Scholar]
- Fiez JA, Damasio H, & Grabowski TJ (2000). Lesion segmentation and manual warping to a reference brain: intra- and interobserver reliability. Hum Brain Mapp, 9(4), 192–211. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10770229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM (1997). The prefrontal cortex: Anatomy, physiology, and neuropsychology of the frontal lobe. Philadelphia: Lippincott-Raven. [Google Scholar]
- Geschwind N (2009). Personality changes in temporal lobe epilepsy. Epilepsy & Behavior, 15(4), 425–433. doi: 10.1016/j.yebeh.2009.04.030 [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, & Burgess PW (2006). Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. Journal of cognitive neuroscience, 18(6), 932–948. doi: 10.1162/jocn.2006.18.6.932 [DOI] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, . . . Tranel D (2012). Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proceedings of the National Academy of Sciences, 109(36), 14681–14686. doi: 10.1073/pnas.1206608109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, . . . Jenkinson M (2016). A multi-modal parcellation of human cerebral cortex. Nature, 536(7615), 171–178. doi: 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy O (2003). Frontal syndrome and disorders of executive functions. Journal of neurology, 250(1), 1–6. doi: 10.1007/s00415-003-0918-2 [DOI] [PubMed] [Google Scholar]
- Godefroy O, Azouvi P, Robert P, Roussel M, LeGall D, Meulemans T, & Group G. d. R. s. l. E. d. F. E. S. (2010). Dysexecutive syndrome: diagnostic criteria and validation study. Annals of neurology, 68(6), 855–864. doi: 10.1002/ana.22117 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1992). Working memory and the mind. Scientific American, 267(3), 110–117. doi: 10.1038/scientificamerican0992-110 [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, & Salazar AM (1996). Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology, 46(5), 1231–1231. doi: 10.1212/WNL.46.5.1231 [DOI] [PubMed] [Google Scholar]
- Harlow JM (1868). Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society, 2, 327–346. doi: 10.1177/0957154X9300401407 [DOI] [Google Scholar]
- Hindman J, Bowren MD, Bruss J, Wright B, Geerling JC, & Boes AD (2018). Thalamic strokes that severely impair arousal extend into the brainstem. Annals of neurology, 84(6), 926–930. doi: 10.1002/ana.25377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M (2013). The human frontal lobes and frontal network systems: an evolutionary, clinical, and treatment perspective. ISRN neurology, 2013. doi: 10.1155/2013/892459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock P, & Polkey C (2003). Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain, 126(7), 1691–1712. doi: 10.1093/brain/awg168 [DOI] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, & Wade D (1996). Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia, 34(4), 247–261. doi: 10.1016/0028-3932(95)00106-9 [DOI] [PubMed] [Google Scholar]
- Husain M, & Roiser JP (2018). Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nature reviews neuroscience, 19(8), 470–484. doi: 10.1038/s41583-018-0029-9 [DOI] [PubMed] [Google Scholar]
- Hwang K, Bruss J, Tranel D, & Boes AD (2020). Network localization of executive function deficits in patients with focal thalamic lesions. Journal of cognitive neuroscience, 32(12), 2303–2319. doi: 10.1162/jocn_a_01628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM. (2020). IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp. [Google Scholar]
- Jenkins LM, Andrewes DG, Nicholas CL, Drummond KJ, Moffat BA, Phal PM, & Desmond P (2018). Emotional reactivity following surgery to the prefrontal cortex. Journal of neuropsychology, 12(1), 120–141. doi: 10.1111/jnp.12110 [DOI] [PubMed] [Google Scholar]
- King ML, Manzel K, Bruss J, & Tranel D (2020). Neural correlates of improvements in personality and behavior following a neurological event. Neuropsychologia, 145. doi: 10.1016/j.neuropsychologia.2017.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleist K (1934). Kriegverletzungen des Gehirns in ihrer Bedeutung für Hirnlokalisation und Hirnpathologie [Significance of war injuries of the brain for brain localization and pathology]. Leipzig, Germany: Barth. [Google Scholar]
- Koziol LF, & Budding DE (2009). Subcortical structures and cognition: Implications for neuropsychological assessment. New York: Springer. [Google Scholar]
- Kretschmer E (1956). Lokalisation und Beurteilung psychophysischer Syndrome bei Hirnverletzten [Localization and evaluation of brain injuries and psychophysical syndromes]. In Rehwald E (Ed.), Das Hirntrauma (pp. 155–158). Stuttgart, Germany: Theime. [Google Scholar]
- Lane-Brown AT, & Tate RL (2009). Apathy after acquired brain impairment: a systematic review of non-pharmacological interventions. Neuropsychological Rehabilitation, 19(4), 481–516. doi: 10.1080/09602010902949207 [DOI] [PubMed] [Google Scholar]
- Laplane D, Degos JD, Baulac M, & Gray F (1981). Bilateral infarction of the anterior cingulate gyri and of the fornices: report of a case. Journal of the neurological sciences, 51(2), 289–300. doi: 10.1016/0022-510x(81)90107-6 [DOI] [PubMed] [Google Scholar]
- Levine B, Schweizer TA, O’Connor C, Turner G, Gillingham S, Stuss DT, . . . Robertson IH (2011). Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Frontiers in human neuroscience, 5, 9. doi: 10.3389/fnhum.2011.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD (1989). Assessment of psychosocial dysfunctions resulting from head trauma. In Lezak MD (Ed.), Assessment of the behavioral consequences of head trauma ((Vol. 7, pp. 113–143). New York: Alan R. Liss. [Google Scholar]
- Logue V, Durward M, Pratt RTC, Piercy M, & Nixon WLB (1968). The quality of survival after rupture of an anterior cerebral aneurysm. The British Journal of Psychiatry, 114(507), 137–160. doi: 10.1192/bjp.114.507.137 [DOI] [PubMed] [Google Scholar]
- Luria AR (1969). Frontal lobe syndromes. In Vinken PJ & Bruyn GW (Eds.), Handbook of clinical neurology: Localization in clinical neurology (Vol. 2). Amsterdam, Netherlands: Noth Holland. [Google Scholar]
- Mahoney CJ, Rohrer JD, Omar R, Rossor MN, & Warren JD (2011). Neuroanatomical profiles of personality change in frontotemporal lobar degeneration. The British Journal of Psychiatry, 198(5), 365–372. doi: 10.1192/bjp.bp.110.082677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinaud O, Perin B, Gérardin E, Proust F, Bioux S, Gars DL, . . . Godefroy O (2009). Anatomy of executive deficit following ruptured anterior communicating artery aneurysm. European Journal of Neurology, 16(5), 595–601. doi: 10.1111/j.1468-1331.2009.02546.x [DOI] [PubMed] [Google Scholar]
- McKinlay W, & Brooks D (1984). Methodological problems in assessing psychosocial recovery following severe head injury. Journal of Clinical and Experimental Neuropsychology, 6(1), 87–99. doi: 10.1080/01688638408401199 [DOI] [PubMed] [Google Scholar]
- Milner B, Petrides M, & Smith ML (1985). Frontal lobes and the temporal organization of memory. Human neurobiology, 4(3), 137. [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Bramati IE, Krueger F, Tura B, . . . Grafman J (2011). Impairment of prosocial sentiments is associated with frontopolar and septal damage in frontotemporal dementia. Neuroimage, 54(2), 1735–1742. doi: 10.1016/j.neuroimage.2010.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders P, Llera A, Tendolkar I, van Eijndhoven P, & Beckmann C (2018). Personality profiles are associated with functional brain networks related to cognition and emotion. Scientific reports, 8(1), 1–8. doi: 10.1038/s41598-018-32248-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JM, & Jacobs LL (1951). Bilateral lesions of the anterior cingulate gyri; report of case. Bulletin of the Los Angeles Neurological Society, 16(2), 231. [PubMed] [Google Scholar]
- Pandya DN, & Barnes CL (1987). Architecture and connections of the frontal lobe. In Perecman E (Ed.), The frontal lobes revisited (pp. 41–72). New York, NY: IRBN Press. [Google Scholar]
- Pandya DN, & Yeterian EH (1996). Comparison of prefrontal architecture and connections. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 351(1346), 1423–1432. doi: 10.1098/rstb.1996.0127 [DOI] [PubMed] [Google Scholar]
- Parvizi J, Rangarajan V, Shirer WR, Desai N, & Greicius MD (2013). The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron, 80(6), 1359–1367. doi: 10.1016/j.neuron.2013.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, & Pandya DN (1994). Comparative architectonic analysis of the human and macaque frontal cortex. In Boller F & Grafman J (Eds.), Handbook of Neuropsychology (Vol. 9, pp. 17–57). Amsterdam: Elsevier. [Google Scholar]
- Phelps C (1897). Traumatic Injuries of the Brain and its Membranes (Vol. 28). New York, New York: Appleton & Co. [Google Scholar]
- Phillips LH, MacPherson SE, & Della Sala S (2002). Age, cognition and emotion: The role of anatomical segregation in the frontal lobes. In Grafman J (Ed.), The frontal lobes (2nd ed., Vol. 7, pp. 73–98). Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Pustina D, Avants B, Faseyitan OK, Medaglia JD, & Coslett HB (2018). Improved accuracy of lesion to symptom mapping with multivariate sparse canonical correlations. Neuropsychologia, 115, 154–166. doi: 10.1016/j.neuropsychologia.2017.08.027 [DOI] [PubMed] [Google Scholar]
- Robinson H, Calamia M, Gläscher J, Bruss J, & Tranel D (2014). Neuroanatomical correlates of executive functions: a neuropsychological approach using the EXAMINER battery. Journal of the International Neuropsychological Society: JINS, 20(1), 52. doi: 10.1017/s135561771300060x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, & McGrath J (1994). Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery & Psychiatry, 57(12), 1518–1524. doi: 10.1136/jnnp.57.12.1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe AD, Bullock PR, Polkey CE, & Morris RG (2001). “Theory of mind” impairments and their relationship to executive functioning following frontal lobe excisions. Brain, 124(3), 600–616. doi: 10.1093/brain/124.3.600 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens T, Rudebeck P, & Walton M (2007). Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends in cognitive sciences, 11(4), 168–176. doi: 10.1016/j.tics.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Ryff CD (1989). Happiness is everything, or is it? Explorations on the meaning of psychological well-being. Journal of personality and social psychology, 57(6), 1069. doi: 10.1037/0022-3514.57.6.1069 [DOI] [Google Scholar]
- Rylander G (1939). Personality changes after operations on the frontal lobes: A clinical study of 32 cases. Copenhagen, Denmark: Munksgaard. [Google Scholar]
- Sanides F (1964). Structure and function of the human frontal lobe. Neuropsychologia, 2(3), 209–219. doi: 10.1016/0028-3932(64)90005-3 [DOI] [Google Scholar]
- Santangelo G, Garramone F, Baiano C, D’Iorio A, Piscopo F, Raimo S, & Vitale C (2018). Personality and Parkinson’s disease: a meta-analysis. Parkinsonism & Related Disorders, 49, 67–74. doi: 10.1016/j.parkreldis.2018.01.013 [DOI] [PubMed] [Google Scholar]
- Sarazin M, Pillon B, Giannakopoulos P, Rancurel G, Samson Y, & Dubois B (1998). Clinicometabolic dissociation of cognitive functions and social behavior in frontal lobe lesions. Neurology, 51(1), 142–148. doi: 10.1212/wnl.51.1.142 [DOI] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, . . . Yeo BTT (2018). Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cerebral Cortex, 28(9), 3095–3114. doi: 10.1093/cercor/bhx179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer R, Popp K, Jörgens S, Lindenberg R, Franz M, & Seitz RJ (2007). Alexithymia-like disorder in right anterior cingulate infarction. Neurocase, 13(3), 201–208. doi: 10.1080/13554790701494964 [DOI] [PubMed] [Google Scholar]
- Schwarz N (1999). Self-reports: How the questions shape the answers. American Psychologist, 54(2), 93. doi: 10.1037/0003-066X.54.2.93 [DOI] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, & Perry D (2009). Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132(3), 617–627. doi: 10.1093/brain/awn279 [DOI] [PubMed] [Google Scholar]
- Simon SS, Varangis E, & Stern Y (2020). Associations between personality and whole-brain functional connectivity at rest: Evidence across the adult lifespan. Brain and behavior, 10(2), e01515. doi: 10.1002/brb3.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, . . . Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23 Suppl 1, S208–219. doi: 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Steinman DR, & Bigler ED (1986). Neuropsychological sequelae of ruptured anterior communicating artery aneurysm. The International journal of clinical neuropsychology, 8(3), 135–140. doi: 10.1007/978-3-7091-6327-6_24 [DOI] [Google Scholar]
- Storey PB (1970). Brain damage and personality change after subarachnoid haemorrhage. The British Journal of Psychiatry, 117(537), 129–142. [PubMed] [Google Scholar]
- Stout JC, Ready RE, Grace J, Malloy PF, & Paulsen JS (2003). Factor analysis of the frontal systems behavior scale (FrSBe). Assessment, 10(1), 79–85. doi: 10.1177/1073191102250339 [DOI] [PubMed] [Google Scholar]
- Strub RL (1989). Frontal lobe syndrome in a patient with bilateral globus pallidus lesions. Archives of Neurology, 46(9), 1024–1027. doi: 10.1001/archneur.1989.00520450096027 [DOI] [PubMed] [Google Scholar]
- Stuss DT (1992). Biological and psychological development of executive functions. Brain and cognition, 20(1), 8–23. doi: 10.1016/0278-2626(92)90059-U [DOI] [PubMed] [Google Scholar]
- Stuss DT (2011). Functions of the frontal lobes: relation to executive functions. Journal of the International Neuropsychological Society, 17(5), 759–765. doi: 10.1017/S1355617711000695 [DOI] [PubMed] [Google Scholar]
- Stuss DT, & Alexander MP (2007). Is there a dysexecutive syndrome? Philosophical Transactions of the Royal Society B: Biological Sciences, 362(1481), 901–915. doi: 10.1098/rstb.2007.2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Floden D, Binns MA, Levine B, McIntosh AR, . . . Hevenor SJ (2002). Fractionation and localization of distinct frontal lobe processes: Evidence from focal lesions in humans. In Stuss DT & Knight RT (Eds.), Principles of frontal lobe function (pp. 392–407). New York: Oxford University Press. [Google Scholar]
- Stuss DT, & Benson DF (1984). Neuropsychological studies of the frontal lobes. Psychological bulletin, 95(1), 3. doi: 10.1037/0033-2909.95.1.3 [DOI] [PubMed] [Google Scholar]
- Stuss DT, Gallup GG Jr, & Alexander MP (2001). The frontal lobes are necessary for “theory of mind”. Brain, 124(2), 279–286. doi: 10.1093/brain/124.2.279 [DOI] [PubMed] [Google Scholar]
- Stuss DT, Gow CA, & Hetherington CR (1992). “No longer Gage”: frontal lobe dysfunction and emotional changes. Journal of consulting and clinical psychology, 60(3), 349. doi: 10.1037/0022-006X.60.3.349 [DOI] [PubMed] [Google Scholar]
- Tate RL (1999). Executive dysfunction and characterological changes after traumatic brain injury: two sides of the same coin? Cortex, 35(1), 39–55. doi: 10.1016/S0010-9452(08)70784-6 [DOI] [PubMed] [Google Scholar]
- Tranel D (2009). The Iowa-Benton school of neuropsychological assessment. In Grant I & Adams KM (Eds.), Neuropsychological assessment of neuropsychiatric and neuromedical disorders (3rd ed., pp. 66–83). New York, NY: Oxford University Press. [Google Scholar]
- Tranel D, Damasio H, Denburg NL, & Bechara A (2005). Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain, 128(12), 2872–2881. doi: 10.1093/brain/awh643 [DOI] [PubMed] [Google Scholar]
- Walch R (1956). Über die Aufgaben der Hirnverletztenheime nach dem Bundesversorgungsgesetz. In Rehwald E (Ed.), Das Hirntrauma (pp. 461–468). Stuttgart, Germany: Thieme. [Google Scholar]
- Wilson DH, & Chang AE (1974). Bilateral anterior cingulectomy for the relief of intractable pain. Stereotactic and functional neurosurgery, 36(1), 61–68. doi: 10.1159/000102783 [DOI] [PubMed] [Google Scholar]
- Wise SP (2008). Forward frontal fields: phylogeny and fundamental function. Trends in neurosciences, 31(12), 599–608. doi: 10.1016/j.tins.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, . . . Polimeni JR (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology, 106, 1125–1165. doi: 10.1152/JN.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, & Pardo JV (2002). Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proceedings of the National Academy of Sciences, 99(4), 2450–2454. doi: 10.1073/pnas.042457199 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized study data are accessible from GitHub: https://github.com/barrashj/APD-NACs_study.git, with the exception of MRI data and lesion masks for which access is constrained by institutional policy requiring a signed data use agreement that is designed to ensure the appropriate use of the data for academic and not commercial purposes. The process by which investigators would acquire this data would be to email the corresponding author at joseph-barrash@uiowa.edu.


