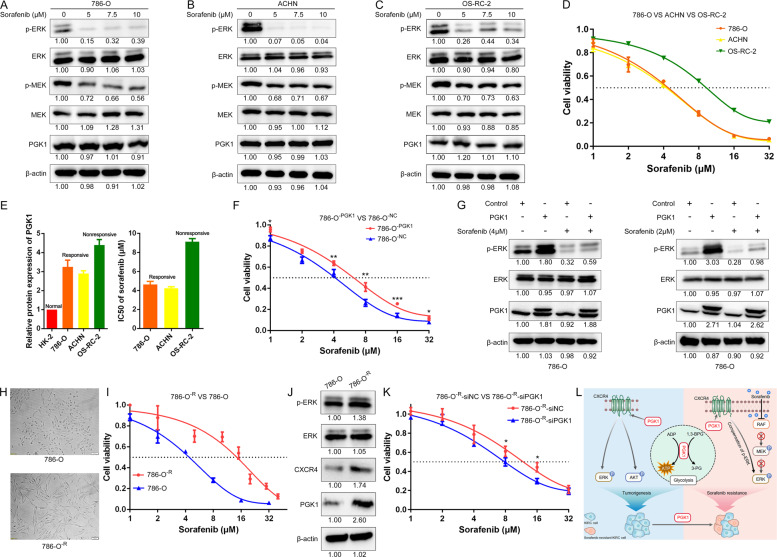
Fig. 8. PGK1 promoted sorafenib resistance via PGK1/CXCR4/ERK signaling.
A–C Sorafenib could inhibit Raf/MEK/ERK signaling pathway in all three KIRC cells. After treatment with sorafenib at the same concentration for the same time, the expression of residual phosphorylated ERK was the highest in OS-RC-2 cells. D CCK8 assays of three KIRC cells after sorafenib treatment at the indicated concentrations for 48 h. E Histograms showing the PGK1 protein expression level and IC50 values (48 h) of three KIRC cells. F CCK-8 assay of PGK1-overexpressing and control 786-O cells after sorafenib treatment at the indicated concentrations for 48 h. G PGK1 promoted sorafenib resistance via increasing ERK phosphorylation in 786-O cells. H Schematic diagram of morphological differences between sorafenib-resistant 786-O cells and wild-type 786-O cells. I CCK-8 assay of sorafenib-resistant 786-O cells after sorafenib treatment at the indicated concentrations for 48 h. J Expression levels of PGK1, CXCR4, ERK and its phosphorylation were detected in sorafenib-resistant 786-O cells and wild-type 786-O cells. K Interference with PGK1 expression in our constructed 786-O-R cells increases their sensitivity to sorafenib. L Schematic diagram of the PGK1-invovled signaling pathway in KIRC progression and sorafenib resistance. In addition to promoting glycolysis to produce ATP for energy supply, PGK1 also induced CXCR4-mediated activation of AKT and ERK phosphorylation to promote tumor growth. Since the RAF/MEK/ERK signaling pathway is one typical target of sorafenib, PGK1 promoted sorafenib resistance via not only accelerating glycolysis but also compensating for the inhibition of ERK phosphorylation caused by sorafenib.