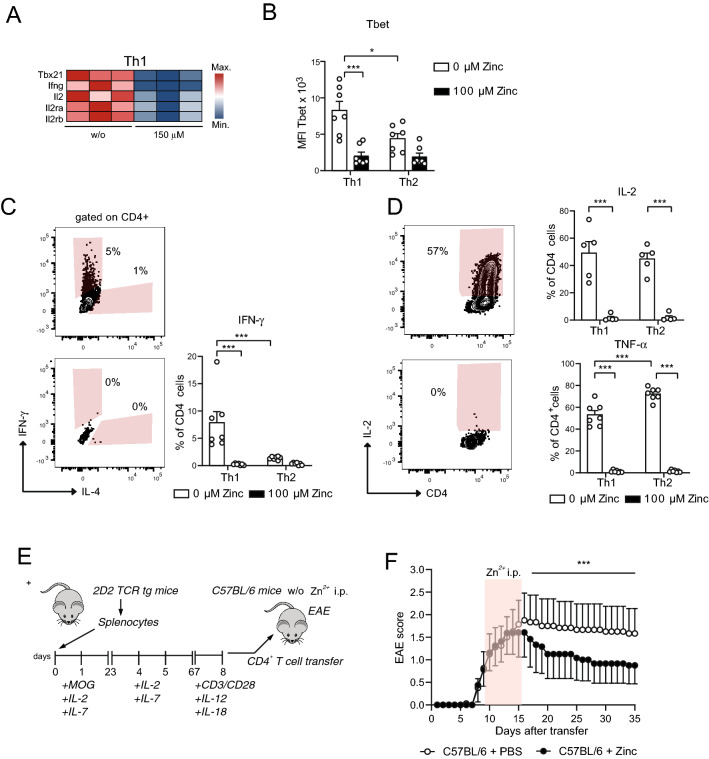
Figure 5.
Zinc aspartate prevents Th1 CNS autoimmunity. (A) Heatmap showing Th1 genes of OT-II TCR tg CD4+ T cells in the absence and presence of 150 μM zinc aspartate. (B) MFI of Tbet measured by FACS of OT-II TCR tg CD4+ T cells differentiated into Th1 and Th2 cells in the absence and presence of 100 μM zinc aspartate. (C) Frequencies of CD4+IFN-γ+ cells measured by FACS after Th1 and Th2 differentiation in the absence and presence of 100 μM zinc aspartate. (D) Frequencies of CD4+IL-2+ and CD4+TNF-α+ cells measured by FACS after Th1 and Th2 differentiation in the absence and presence of 100 μM zinc aspartate. (E) Splenic T cells of 2D2 mice were stimulated in vitro with MOG35-55 in the presence of IL-2 and IL-7 and were reactivated with plate bound anti-CD3 and anti-CD28 in the presence of IL-12 and IL-18. Fully activated transgenic 2D2 T cells were adoptively transferred into C57BL/6 recipients. Zinc aspartate was administered daily i.p. (15 µg/animal) starting at day 8 after the appearance of first clinical signs. Zinc treatment was continued for 7 days. The control group received PBS. (F) The clinical score of passive EAE was assessed for 35 days after transfer. Data are shown as mean ± SEM. RNA sequencing data in (A) was generated in biological triplicates from 3 mice. Statistical analysis in (B–D) by unpaired student’s t test and in (F) by non-parametric Wilcoxon matched pairs test. Dots represent individual mice used to isolate OT-II TCR tg CD4+ T cells for Th differentiation. Data in (B–D) are from 3 experiments. Data in (F) are from 8 mice per group. *p < 0.05, **p < 0.01, ***p < 0.001.