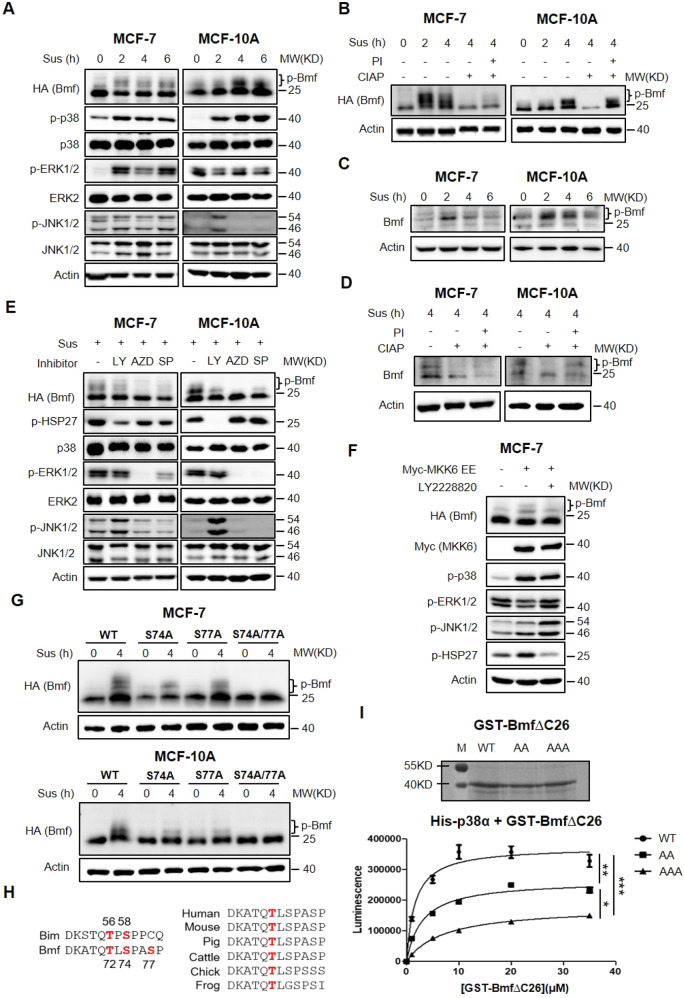
Fig. 2. p38 MAPK phosphorylates Bmf at multiple sites.
A MCF-7 HA-Bmf cells (pre-induced with 100 ng/mL doxycycline for 24 h) and MCF-10A HA-Bmf∆C26 cells (constitutive Bmf expression) were cultured in suspension for 0, 2, 4, 6 h before lysed for western blot analysis. B Lysates of MCF-7 HA-Bmf∆C26 and MCF-10A HA-Bmf∆C26 cells were treated with calf intestine alkaline phosphatase (CIAP) and/or phosphatase inhibitor (PI), followed by western blot analysis. C MCF-7 and MCF-10A cells were cultured in suspension for 0, 2, 4, 6 h and expression of endogenous Bmf was analyzed by western blotting. D MCF-7 and MCF-10A cells were cultured in suspension for 4 h and lysed. Lysates were treated with −/+ CIAP/ PI and expression of endogenous Bmf was analyzed by western blotting. E MCF-7 HA-Bmf cells (pre-treated with 100 ng/mL doxycycline for 24 h) and MCF-10A HA-Bmf∆C26 cells were cultured in suspension in the presence of indicated inhibitors (LY 2228820, 1 μM; AZD6244, 10 μM; SP 600125, 10 μM) for 4 h. Cells were harvested and lysed for western blot analysis. F MCF-7 HA-Bmf∆C26 cells were transfected with empty vector or Myc-MKK6 EE expressing plasmids for 48 h in the presence or absence of p38α inhibitor, LY 2228820 (1 μM). Cells were then lysed for western blot analysis. G MCF-7 or MCF-10A cells transduced with lentiviruses expressing HA-Bmf∆C26 variants (WT, S74A, S77A, S74A/S77A) were individually cultured in suspension for 4 h and lysed for western blot analysis. H Alignment of the DLC1/2 binding domain of Bmf with that of Bim (Upper panel) and among different species (Bottom panel). I In vitro kinase assay was carried out using purified GST-Bmf∆C26 proteins and activated recombinant His-p38α. Top: SDS PAGE of purified GST-Bmf∆C26 and its mutant analogues. Bottom: quantitation of the luminescence as an indicator of phosphorylation level. Quantitative results were shown as mean ± STD from three independent experiments. Significance was determined by ANOVA one-way test, *p < 0.05, **p < 0.01, ***p < 0.001.