Abstract
Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) are present in human umbilical connective tissue and can differentiate into various cell types. Our previous studies have proved that hUC-MSCs do not lead to allergies and tumorigenesis. In the present study, the acute and long-term toxicity of hUC-MSCs in mice and rats was evaluated. The acute toxicity of hUC-MSCs was assessed in 8-week-old mice receiving two caudal intravenous (i.v.) injections of hUC-MSCs at the maximum tolerated dose of 1.5 × 107 cells/kg with an interval of 8 h and the observation period sustained for 14 days. For the long-term toxicity evaluation, rats were randomly divided into control, low-dose (3.0 × 105 cells/kg), mid-dose (1.5 × 106 cells/kg), and high-dose (7.5 × 106 cells/kg) groups, which were treated with hUC-MSCs via a caudal i.v. injection every 3 days for 90 days. Weight and food intake evaluation was performed for all rats for 2 weeks after the hUC-MSC administration. The animals were then sacrificed for hematological, blood biochemical, and pathological analyses, as well as organ index determination. We observed no obvious acute toxicity of hUC-MSCs in mice at the maximum tolerated dose. Long-term toxicity tests in rats showed no significant differences between HUC-MSC-treated and control groups in the following parameters: body weight, hematological and blood biochemical parameters, and histopathologic changes in the heart, liver, kidneys, and lungs. This study provides evidence of the safety of i.v. hUC-MSCs infusion for future clinical therapies.
Keywords: Acute toxicity, Biotransformation and toxicokinetics, Human umbilical cord-derived mesenchymal stem cells, Long-term toxicity, Safety evaluation
Introduction
Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) are present in human umbilical connective tissue and can differentiate into various cell types (Jia et al. 2017; Yousefi et al. 2017; Bae et al. 2018). Therefore, they are currently considered an ideal seed cell for treating cell damage or loss associated with certain diseases.
MSCs are derived from the mesoderm and ectoderm in the early stage of embryonic development. MSCs are able to maintain their multiple differential potential after passage culture or frozen storage (Mussano et al. 2018; Real et al. 2017; Gómez-Aristizábal et al. 2017). Their immunomodulatory nature (Zhang and He 2019), hematopoietic support, and tissue repair (Li et al. 2019), make MSCs ideal seed cells for treatment of diseases, aging, and suboptimal health.
MSCs bear great potential for the clinical treatment of multiple organ injuries due to their convenient and extensive source availability, sufficient quantities, low immunogenicity (Devito et al. 2019; Dong et al. 2019), lack of ethical controversy, fast proliferation (Harrell et al. 2019), strong differentiation ability, and extensive application prospects (Phonchai et al. 2019; Lin et al. 2019; Han et al. 2018). However, determination of their safety is now required for clinical applications. Our previous studies have proved that hUC-MSCs do not lead to allergies (Jianwei et al. 2014) and tumorigenesis (Jianwei et al. 2015; Ryan et al. 2005), but the acute and chronic toxicity of hUC-MSCs has not yet been tested. In the present study, the acute and long-term toxicity of hUC-MSCs was evaluated. This study provides a theoretical basis for the safety of hUC-MSC intravenous (i.v.) infusion in future clinical therapies.
Materials and methods
Materials
All the experiments were approved by the Human Ethics Committee of the Affiliated Hospital of Guizhou Medical University and the Human Ethics Committee of Soochow University.
Neonatal umbilical cords were provided by the obstetrical department of the Affiliated Hospital of Guizhou Medical University. Low-glucose Dulbecco’s Modified Eagle’s medium (DMEM) and serum-free medium were purchased from Gibco (USA). Fetal bovine serum (FBS) and 0.25% trypsin–EDTA were purchased from Hyclone (USA). Instruments used in the study included a phase-contrast microscope (PANASONIC, Japan), CO2 incubator (Forma Scientific, USA), and flow cytometer (MOFLO High Performance Cell Sorter, BECKMAN COULTER, USA). hUC-MSC suspensions were prepared by the Tissue Engineering and Stem Cell Research Center of Guizhou Medical University. 8-week-old female Balb/c nude mice,8-week-old Kunming mice and 3-week-old male Sprague–Dawley rats were housed in a specific pathogen free (SPF) grade facility provided by the Animal Lab Center of Guizhou Medical University. The animal quality certificate number for the study was SCXK (Qian) 2012-001.
Culture and identification of hUC-MSCs. Separation, cultivation, and amplification of hUC-MSCs
Human primary MSCs were obtained from neonatal umbilical cords. The umbilical cords were first placed on a clean bench and residual blood was thoroughly washed off with saline, excluding the inner and outer membranes of the vascular tissue (umbilical artery and vein). The cells were isolated from the Wharton’s jelly, which was stripped off the cords and cut into 1 mm3 pieces. The collected tissue blocks were then placed in a solution of saline and centrifuged for 3 min at 1200 rpm. After the supernatant was removed, the organization blocks were inoculated on Petri dishes containing DMEM/F12 complete medium with a straw and cultivated at 37 °C and 5% CO2 in a humidity-saturated incubator. Cell growth was observed under an inverted microscope. 50% of the medium was first changed after 3–5 days and then 3 days thereafter. The tissue blocks were removed when cells became 50% confluent and the resulting cells were subcultured until 80%-90% confluency was reached. Passage 4 (P4) cells were then collected, digested into a single-cell suspension, and flow cytometric analysis was performed as described previously.
Flow cytometry identification
After digestion, the hUC-MSC suspension was centrifuged at 1300 rpm for 8 min. Then the cells were resuspended in phosphate buffered saline (PBS) into a single-cell suspension by gentle blowing. Later, 20 μL of mouse anti-human CD44-APC, CD73-PerCP, CD90-PE, and CD105-FITC antibody were added into each tube containing 50 μL of the single-cell suspension and incubated at room temperature for 30 min in the dark. Then, 1 mL of PBS was added into each tube and centrifuged at 1500 rpm for 6 min. After removal of the supernatant, 2 mL of PBS were added into each tube and centrifuged again at 1500 rpm for 6 min. After the supernatants were discarded, the cell pellets were finally resuspended in 400 μL of PBS and detected by flow cytometry.
Determination of acute toxicity
Determination of median lethal dose (LD50) for acute toxicity test
Eighty KM mice (SPF grade; 20–22 g; 40 males and 40 females) were randomly divided into four groups (low-dose, mid-dose, high-dose, and control). Digested P4 hUC-MSCs were washed three times and then resuspended in saline to amount to a total volume of 0.5 mL. All animals were injected once with different doses of the cell suspension (3.0 × 105 cells/kg, 1.5 × 106 cells/kg, and 7.5 × 106 cells/kg) or saline solution with the same volume. After HUC-MSC administration, all of the animals were kept under continuous observation and any changes in behavior or physical activity were recorded daily for 7 days. At the end of the experiment, all surviving mice were anesthetized with phenobarbital (0.4%) and autopsied to evaluate and record whether there were any obvious changes in major organs. If no animal deaths occurred, the maximum tolerated dose (MTD) was measured.
Determination of MTD for acute toxicity testing
Forty KM mice (8 weeks old; 20 ± 2 g; 20 males and 20 females; fed freely) were randomly divided into two groups. The mice underwent two i.v. injections of either a 7.5 × 106 cells/kg hUC-MSC solution or saline with an interval of 8 h. After the HUC-MSC administration, general mouse health symptoms, weight changes, and mortality were observed and recorded daily for 14 days. At the end of the experiment, all surviving mice were anesthetized with pentothal sodium phenobarbital (0.4%) and autopsied.
Determination of long-term toxicity
Sixty four Sprague Dawley (SD) rats (200 ± 20 g; 32 males and 32 females) were randomly divided into four groups and acclimated for at least 2 weeks before use. The control group received i.v. injections of saline and three treatment groups received i.v. injections of hUC-MSC suspensions with different doses (3.0 × 105 cells/kg, 1.5 × 106 cells/kg, and 7.5 × 106 cells/kg) weekly for 12 weeks. The animals’ appearance, behavior, food and water intake, and skin, hair, and eyelid condition, as well as motion quality, behavior, algesia, stool, and urination were observed daily. The body weight of each animal was recorded weekly and the differences among groups were compared. All of the SD rats were anesthetized 14 days after the last treatment was completed. Their blood samples were collected from the femoral artery for hematology and coagulation tests. At the end of the experiment, all of the rats were sacrificed and autopsied. The heart, brain, liver, spleen, lungs, kidneys, bone marrow, and ovaries/testicles of the animals from different groups were extracted, weighed, and preserved in a 10% formalin solution. Hematoxylin and eosin and immunohistochemical staining was performed using standard protocols and the results were evaluated using an inverted microscope. The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies (Tveden-Nyborg et al. 2018; Hakkola et al. 2018).
Tumorigenicity test of hUC-MSCs
Ten Balb/c nude mice (SPF grade; 18–20 g; female) were randomly divided into two groups (control group and experimental group). Animals are raised under the condition of independent ventilation cage (IVC), The experimental mice were hypodermic injected with P4 hUC-MSCs 1 × 109/L cell suspension 0.5 mL on the inner side of the unilateral front and hindleg and the control mice were injected with the same amount of saline at the same site. The growth of tumor and other adverse reactions in nude mice were observed within 8 weeks, and the nude mice were killed at the end of 8 weeks after injection, and routine histopathological examination was performed.
Statistical analysis
Measurement results were reported as mean ± standard deviation. Data among groups were compared using one-way analysis of variance (ANOVA). p < 0.05 was considered to be statistically significant. All analyses were conducted using the SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
Isolation, culture, amplification, and identification of hUC-MSCs
Isolation, culture and amplification of hUC-MSCs
Observation of morphological MSC properties is the most intuitive method for clearly differentiating cell types. After the initial 7 days of culture, the cells migrated adherently from the umbilical cord tissue. After 14 days, the cell confluency reached 50%. After the tissues were removed, cells grew rapidly and their density reached 80–90% within 20 days (Fig. 1A). After the first passage, the medium was replaced by serum-free medium containing growth factors and the cells grew more rapidly and appeared as long spindle-shaped fibroblasts or polygonal cells. hUC-MSCs were passaged at a split ratio of 1:2, and became confluent and ready to be passaged further after 3–5 days. A large number of hUC-MSCs can then be purified and amplified by passaging to meet particular clinical needs.
Fig. 1.
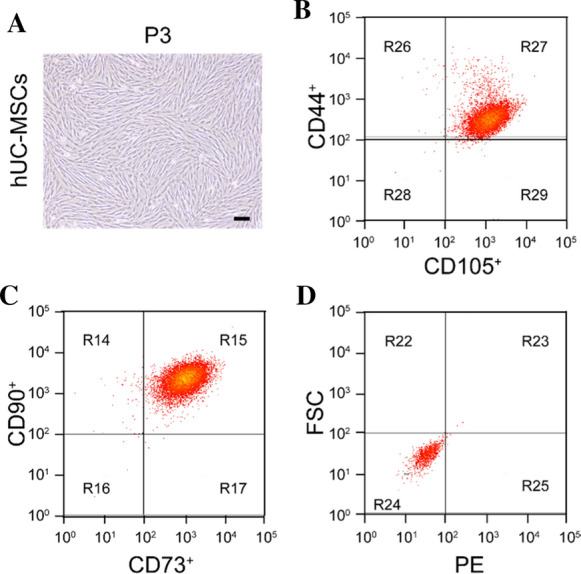
Culture and identification of hUC-MSCs. hUC-MSCs were cultured with FBS for third passage. A Diagram of the third passage hUC-MSCs. B–D CD105, CD44, CD73 and CD90 were determined using flow cytometry and were highly expressed, with almost no expression on NON-hUC-MSCs Bar = 100 μm
hUC-MSC phenotype determination
The flow detection method was used to identify the cultured P3 cell surface antigen markers. Flow cytometry showed that the hUC-MSC-specific markers CD90, CD44, CD105, and CD73 were highly expressed (Fig. 1B–D), with almost no expression on NON-hUC-MSCs.
Toxicological evaluation studies in mice
LD50 for acute toxicity
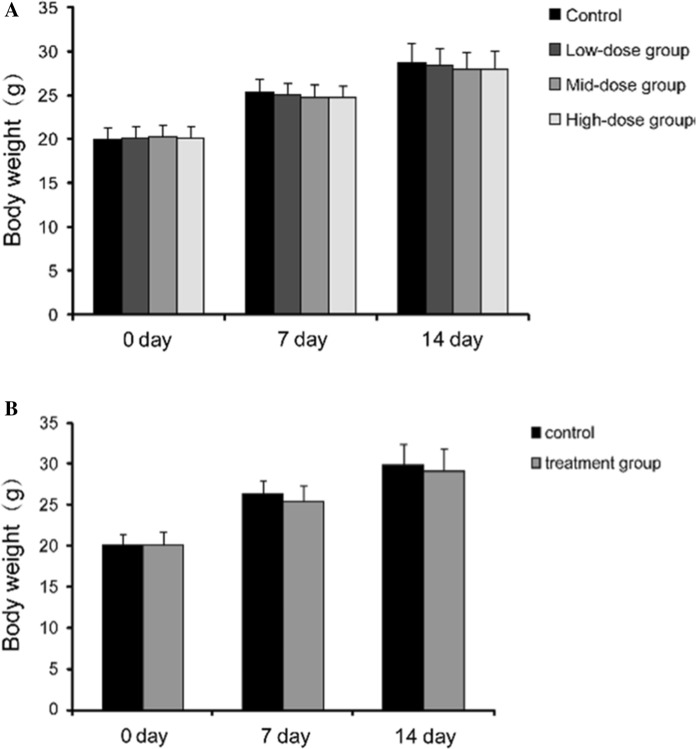
Our observations revealed that the KM mice survived and behaved normally in the 7 days after administration of the hUC-MSCs suspensions. Body weights of both female and male mice increased at a normal rate (Fig. 2A). hUC-MSCs were not found to be toxic, nor were they found to cause mortality. Non-significant changes were observed in the wellness parameters used for evaluation of toxicity.
Fig. 2.
Changes of body weight in mice with acute toxicity test of hUC-MSCs. A There was no significant difference among the hUC-MSCs treatment groups (low-dose group, mid-dose group and high-dose group) or the control group at the indicated time points (p > 0.05). B Body weight changes of mice i.v. injected hUC-MSCs at MTD. There was no significant difference between the control group and the treatment group at the indicated time points (p > 0.05)
MTD for acute toxicity
After the i.v. injection of hUC-MSCs (1.5 × 107 cells/kg), treatment group mice became quiet and their activity decreased. They gradually recovered over the course of 20 min. The animals’ hair in both groups was smooth and their behavior, food consumption, and excretion were normal during the observation period. There were no significant differences in weight between the hUC-MSCs-treated and control mice (Fig. 2B). Autopsy results revealed that no changes were observed in organ structure and no mortality was recorded during the observation period.
The mice were treated with hUC-MSCs at the MTD of 1.5 × 107 cells/kg, which is equivalent to 50–100 times of the clinical adult dose. These data demonstrated that i.v. injection of hUC-MSCs was safe.
Long-term toxicity of hUC-MSCs
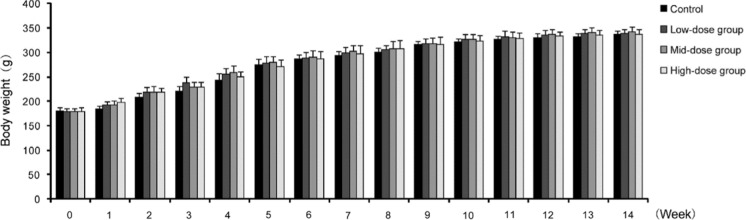
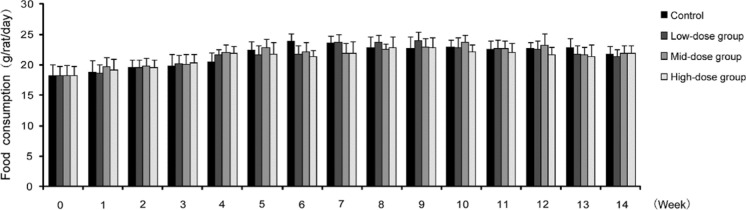
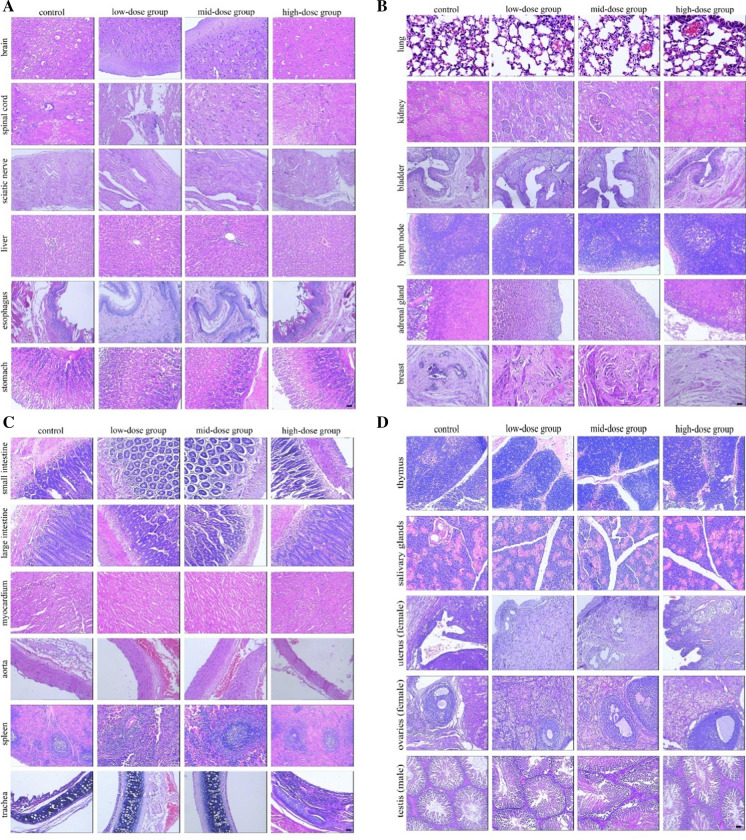
All of the animals, including the hUC-MSC-treated and control groups, showed no marked abnormalities during the study. We found that they displayed normal characteristics that included normal behavior, smooth hair, absence of abnormal secretions from the urethra or anus, and no death. In addition, the body weight, dietary consumption, and organ index (organ-to-body weight ratio) of rats showed no significant differences (Fig. 3, 4, Table 1). Hematology, blood biochemistry, and blood coagulation were assessed and no obvious abnormalities were observed in the rats treated with hUC-MSCs compared to the control group (Tables 2, 3, Fig. 5). Histological examination of the rats’ major organs was also conducted in this study. Compared to the control group, no significant differences were observed in the organs of rats treated with different dosages of hUC-MSCs (Fig. 6).
Fig. 3.
Changes of body weight in long-term toxicity test of hUC-MSCs in rats. There was no significant difference among the hUC-MSCs treatment groups (low-dose group, mid-dose group and high-dose group) or the control group at the indicated time points (p > 0.05)
Fig. 4.
Changes of dietary consumption in long-term toxicity test of hUC-MSCs in rats. There was no significant difference among the hUC-MSCs treatment groups (low-dose group, mid-dose group and high-dose group) or the control group at the indicated time points (p > 0.05)
Table 1.
Changes of organ index in long-term toxicity test of hUC-MSCs in rats (%, )
| Viscera | Control | Low-dose group | Mid-dose group | High-dose group | |
|---|---|---|---|---|---|
| 12 week | Heart | 1.54 ± 0.16 | 1.31 ± 0.17 | 1.48 ± 0.21 | 1.67 ± 0.45 |
| Liver | 10.02 ± 2.46 | 12.90 ± 3.61 | 15.21 ± 3.79 | 13.97 ± 2.89 | |
| Spleen | 0.73 ± 0.12 | 0.71 ± 0.13 | 0.92 ± 0.17 | 0.83 ± 0.15 | |
| Lung | 2.03 ± 0.53 | 2.24 ± 0.37 | 1.98 ± 0.33 | 2.64 ± 0.44 | |
| Kidney | 3.65 ± 0.54 | 3.76 ± 0.28 | 2.64 ± 0.38 | 3.21 ± 0.27 | |
| Brain | 2.20 ± 0.11 | 2.01 ± 0.30 | 2.17 ± 0.23 | 2.14 ± 0.31 | |
| Renicapsule | 0.08 ± 0.02 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.7 ± 0.02 | |
| Thymus | 0.24 ± 0.08 | 0.27 ± 0.07 | 0.30 ± 0.07 | 0.45 ± 0.17 | |
| Uterus | 0.67 ± 0.15 | 0.74 ± 0.21 | 0.86 ± 0.18 | 0.75 ± 0.13 | |
| Ovary | 0.19 ± 0.01 | 0.16 ± 0.03 | 0.15 ± 0.03 | 0.17 ± 0.05 | |
| Testis epididymis | 4.49 ± 0.71 | 4.16 ± 0.68 | 4.75 ± 0.83 | 4.79 ± 0.74 | |
| 14 week | Heart | 1.78 ± 0.26 | 1.59 ± 0.35 | 1.77 ± 0.32 | 1.66 ± 0.39 |
| Liver | 12.23 ± 3.79 | 14.91 ± 4.11 | 11.86 ± 3.21 | 13.68 ± 3.31 | |
| Spleen | 0.95 ± 0.15 | 0.91 ± 0.19 | 0.81 ± 0.16 | 0.71 ± 0.13 | |
| Lung | 2.83 ± 0.73 | 2.05 ± 0.34 | 1.38 ± 0.23 | 1.94 ± 0.27 | |
| Kidney | 2.15 ± 0.43 | 3.33 ± 0.39 | 3.24 ± 0.68 | 2.92 ± 0.43 | |
| Brain | 2.24 ± 0.31 | 2.21 ± 0.22 | 2.35 ± 0.48 | 2.41 ± 0.25 | |
| Renicapsule | 0.08 ± 0.01 | 0.07 ± 0.02 | 0.08 ± 0.01 | 0.8 ± 0.02 | |
| Thymus | 0.40 ± 0.15 | 0.29 ± 0.11 | 0.39 ± 0.07 | 0.39 ± 0.34 | |
| Uterus | 0.75 ± 0.18 | 0.70 ± 0.19 | 0.78 ± 0.13 | 0.77 ± 0.15 | |
| Ovary | 0.14 ± 0.04 | 0.16 ± 0.05 | 0.17 ± 0.03 | 0.16 ± 0.03 | |
| Testis epididymis | 5.19 ± 0.32 | 4.84 ± 0.48 | 4.88 ± 0.63 | 4.91 ± 0.84 | |
12 weeks, n = 10; stop injection 2 weeks, n = 6; each dose group compared with the control group, P > 0.05
There was no significant difference among the hUC-MSCs treatment groups (low-dose group, mid-dose group and high-dose group) the heart, brain, liver, spleen, lungs, kidneys, bone marrow, and ovaries/testicles of the animals or the control group at the indicated time points (p > 0.05)
Table 2.
Changes of blood routine in long-term toxicity test of hUC-MSCs in rats ()
| Name | Control | Low-dose group | Mid-dose group | High-dose group | |
|---|---|---|---|---|---|
| 12 week | WBC (G/L) | 6.74 ± 2.41 | 8.30 ± 2.38 | 5.65 ± 2.41 | 7.43 ± 3.13 |
| NUET (%) | 10.80 ± 2.82 | 13.17 ± 3.24 | 12.68 ± 3.73 | 10.79 ± 3.25 | |
| LYMPH (%) | 73.06 ± 9.26 | 76.54 ± 7.21 | 77.31 ± 7.37 | 67.25 ± 7.83 | |
| RBC (T/L) | 7.11 ± 1.25 | 7.03 ± 0.83 | 6.29 ± 1.01 | 6.61 ± 0.77 | |
| HGB (g/L) | 145.57 ± 12.71 | 147.61 ± 12.56 | 151.11 ± 11.71 | 149.38 ± 11.22 | |
| HCT (%) | 41.56 ± 6.14 | 42.10 ± 5.29 | 46.41 ± 4.96 | 45.78 ± 5.36 | |
| PLT (G/L) | 796.16 ± 246.42 | 696.52 ± 253.14 | 726.82 ± 210.35 | 790.23 ± 261.40 | |
| RETIC (G/L) | 217.04 ± 76.38 | 210.40 ± 87.04 | 197.85 ± 69.68 | 215.48 ± 70.85 | |
| 14 week | WBC (G/L) | 7.71 ± 2.31 | 7.20 ± 2.18 | 6.78 ± 2.31 | 7.38 ± 3.18 |
| NUET (%) | 11.21 ± 2.32 | 12.96 ± 3.14 | 12.88 ± 3.56 | 11.59 ± 3.34 | |
| LYMPH (%) | 71.48 ± 6.68 | 73.64 ± 7.73 | 79.31 ± 7.87 | 70.98 ± 7.29 | |
| RBC (T/L) | 6.91 ± 1.75 | 7.55 ± 0.76 | 7.32 ± 1.04 | 6.87 ± 0.45 | |
| HGB (g/L) | 120.83 ± 10.21 | 136.68 ± 11.54 | 160.34 ± 13.31 | 143.47 ± 11.60 | |
| HCT (%) | 48.61 ± 6.32 | 43.89 ± 5.56 | 49.51 ± 4.68 | 42.38 ± 3.96 | |
| PLT (G/L) | 906.32 ± 296.12 | 778.32 ± 238.49 | 686.91 ± 247.47 | 850.41 ± 281.51 | |
| RETIC (G/L) | 197.09 ± 86.59 | 232.06 ± 91.34 | 247.12 ± 75.69 | 175.36 ± 61.34 | |
12 week, n = 10; 14 week, n = 6; each dose group compared with the control group, P > 0.05
There was no significant difference among the hUC-MSCs treatment groups (low-dose group, mid-dose group and high-dose group) blood routine indexes or the control group at the indicated time points (p > 0.05)
Table 3.
Changes of Biochemical index in long-term toxicity test of hUC-MSCs in rats ()
| Name | Control | Low-dose group | Mid-dose group | High-dose group |
|---|---|---|---|---|
| A | ||||
| K (mmol/L) | 6.12 ± 0.11 | 6.27 ± 0.55 | 6.78 ± 0.48 | 7.03 ± 0.25 |
| Na (mmol/L) | 147.22 ± 2.77 | 149.16 ± 3.86 | 142.71 ± 3.63 | 143.58 ± 3.39 |
| Cl (mmol/L) | 105.21 ± 2.51 | 108.78 ± 2.56 | 111.25 ± 2.98 | 115.18 ± 2.74 |
| BUN (mmol/L) | 4.32 ± 0.33 | 6.27 ± 1.29 | 5.85 ± 1.64 | 6.62 ± 1.43 |
| CREA (umol/L) | 50.11 ± 6.88 | 42.27 ± 6.47 | 53.63 ± 5.11 | 47.50 ± 5.80 |
| GLU (mmol/L) | 3.86 ± 0.52 | 4.99 ± 0.56 | 5.38 ± 0.19 | 5.85 ± 0.63 |
| ALT (U/L) | 50.21 ± 8.76 | 68.56 ± 7.44 | 60.23 ± 10.35 | 58.66 ± 7.27 |
| AST (IU/L) | 275.56 ± 11.10 | 298.54 ± 21.34 | 229.54 ± 18.16 | 248.56 ± 13.45 |
| TBIL (umol/L) | 1.34 ± 0.32 | 1.31 ± 0.25 | 2.01 ± 0.23 | 2.12 ± 0.24 |
| TP (g/L) | 75.58 ± 5.37 | 88.32 ± 6.67 | 84.11 ± 5.78 | 89.15 ± 6.28 |
| ALB (g/L) | 31.25 ± 2.44 | 40.18 ± 2.66 | 47.54 ± 1.59 | 38.04 ± 2.87 |
| GLB (g/L) | 32.98 ± 2.56 | 38.68 ± 1.59 | 40.29 ± 2.32 | 38.37 ± 2.47 |
| ALP (U/L) | 89.11 ± 18.26 | 87.87 ± 14.78 | 74.25 ± 12.84 | 82.14 ± 17.55 |
| CK (U/L) | 2240.58 ± 639.87 | 2364.18 ± 484.98 | 2009.54 ± 576.23 | 2168.67 ± 525.46 |
| TG (mmol/L) | 1.56 ± 0.09 | 1.48 ± 0.11 | 1.34 ± 0.13 | 1.53 ± 0.28 |
| CHOL (mmol/L) | 1.38 ± 0.27 | 1.24 ± 0.23 | 1.73 ± 0.29 | 1.70 ± 0.25 |
|
B Effect of stopping injection of hUC-MSCs on 2 week biochemical Indexes in Rats () |
||||
| K (mmol/L) | 6. 27 ± 0.15 | 6.32 ± 0.34 | 7.58 ± 0.62 | 6.63 ± 0.23 |
| Na (mmol/L) | 144.40 ± 3.75 | 141.86 ± 2.79 | 138.78 ± 3.90 | 149.50 ± 3.10 |
| Cl (mmol/L) | 111.44 ± 2.51 | 112.47 ± 1.39 | 117. 10 ± 2.58 | 112.13 ± 2.65 |
| BUN (mmol/L) | 6.16 ± 0.43 | 7.23 ± 1.10 | 4.75 ± 1.13 | 5.67 ± 1.32 |
| CREA (umol/L) | 41.18 ± 7.89 | 47.55 ± 6.09 | 51.38 ± 5.86 | 45.56 ± 5.37 |
| GLU (mmol/L) | 4.39 ± 0.48 | 4.75 ± 0.32 | 5.65 ± 0.73 | 5.78 ± 0.65 |
| ALT (U/L) | 66.39 ± 8.86 | 65.21 ± 7.83 | 67.54 ± 10.21 | 68.63 ± 10.10 |
| AST (IU/L) | 214.17 ± 16.16 | 235.12 ± 28.38 | 265.43 ± 27.49 | 245.46 ± 19.18 |
| TBIL (umol/L) | 1.49 ± 0.42 | 2.37 ± 0.39 | 3.24 ± 0.65 | 2.32 ± 0.31 |
| TP (g/L) | 78.24 ± 6.68 | 71.11 ± 5.78 | 81.62 ± 6.36 | 80.22 ± 7.58 |
| ALB (g/L) | 37.68 ± 2.84 | 42.11 ± 2.80 | 39.91 ± 1.92 | 43.10 ± 2.52 |
| GLB (g/L) | 39.61 ± 2.35 | 40.45 ± 1.66 | 41.41 ± 2.18 | 40.23 ± 1.87 |
| ALP (U/L) | 78.71 ± 14.43 | 80.81 ± 17.17 | 64.01 ± 10.71 | 69.61 ± 14.13 |
| CK (U/L) | 2438.91 ± 663.86 | 2764.67 ± 464.61 | 1909.34 ± 516.72 | 2036.87 ± 577.56 |
| TG (mmol/L) | 1.33 ± 0.11 | 1.29 ± 0.15 | 1.36 ± 0.16 | 1.45 ± 0.34 |
| CHOL (mmol/L) | 1.23 ± 0.20 | 1.42 ± 0.31 | 1.93 ± 0.38 | 1.67 ± 0.29 |
A: 12 week, n = 10; each dose group compared with the control group, P > 0.05
B: 14 week, n = 6; each dose group compared with the control group, P > 0.05
(A) There was no significant difference among the hUC-MSCs treatment groups (low-dose group, mid-dose group and high-dose group) blood routine indexes or the control group at the 12 weeks (p > 0.05). (B) There was no significant difference among the hUC-MSCs treatment groups (low-dose group, mid-dose group and high-dose group) blood routine indexes or the control group at the 14 weeks (p > 0.05)
Fig. 5.
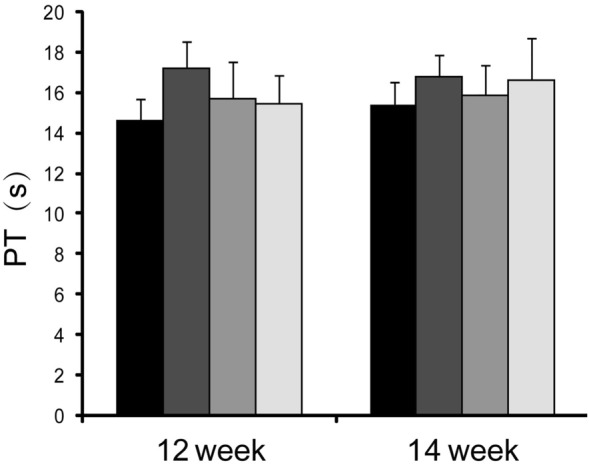
Changes of coagulation function in long-term toxicity test of hUC-MSCs in rats. There was no significant difference among the hUC-MSCs treatment groups (low-dose group, mid-dose group and high-dose group) blood routine indexes or the control group at the indicated time points (p > 0.05)
Fig. 6.
Pathological hematoxylin–eosin staining analysis analyses of the long-term toxicity of hUC-MSCs. Representative images of brain, spinal cord, sciatic nerve, liver, esophagus, stomach, small intestine, large intestine, myocardium, aorta, spleen, trachea, lung, kidney, bladder, lymph node, adrenal gland, breast, thymus, salivary glands, uterus (female), ovaries (female), testis (male). There was no significant difference among the hUC-MSCs treatment groups (low-dose group, mid-dose group and high-dose group) or the solvent control group. Bar = 100 μm
Toxicological evaluation studies of hUC-MSCs
Observation of nude mice after injection of hUC-MSCs
During the 8-week observation period, there was no obvious adverse reaction and no death in nude mice. At the end of the 8th week after injection of hUC-MSCs suspension, no tumor was found after Executed and dissected (Fig. 7). Pathological examination showed normal tissue structure, no colony formation and no special pathological changes.
Fig. 7.
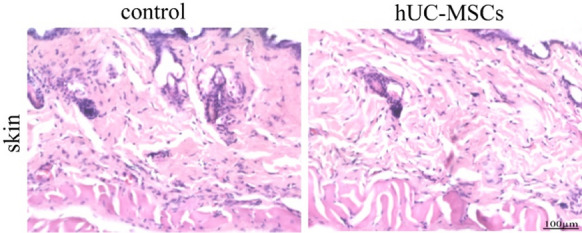
Pathological hematoxylin–eosin staining analysis of the hUC-MSCs injection site in nude mice. There was no significant difference between hUC-MSCs and control group. Bar = 100 μm
Discussion
hUC-MSCs are typical adult stem cells that are characterized by their low immunogenicity, non-invasive harvesting procedure, easy expansion in vitro, and ethical access compared to stem cells from other sources (Zhang et al. 2018; Iaffaldano et al. 2018; Dostert et al. 2017; Zheng et al. 2015). hUC-MSCs seem to be an optimal choice for stem cell-based therapy. Although MSC-based treatments have progressed in recent years, before the cells are fully translated from basic to clinical research, some issues still remain to be solved, especially with regard to their safety (Poon et al. 2019; Fazeli et al. 2018). In this study, we conducted experiments to evaluate the safety of hUC-MSCs (Jianwei et al. 2014, 2015).
Preclinical toxicology studies were performed according to the pharmacological methods, which assess the safety of hUC-MSCs. LD50 is the median lethal dose for 50% of the subjects and is an important metric that reflects the extent of acute toxic response in animals. Our research indicated no statistically significant changes in the body weight, food intake, skin, behavior, secretion, and excretion of the animals in the group undergoing i.v. injections of hUC-MSCs. In addition, no mice died during the treatment and as such, no LD50 could be obtained. Therefore, we evaluated acute toxicity of hUC-MSCs with the MTD test.
The total dosage of administered hUC-MSCs was 1.5 × 107 cells/kg for the two i.v. injections. The mice behaved normally and no death occurred during the observation period. The dosage of hUC-MSCs was equivalent to 50–100 times that of the clinical adult dose, which indicates that the acute toxicity of hUC-MSCs in mice was low and that they are safe seed cells for clinical use. According to the reported protocols and feasibility of experiments, we studied three dose levels: low-dose (3.0 × 105 cells/kg), mid-dose (1.5 × 106 cells/kg), and high-dose (7.5 × 106 cells/kg), which were equivalent to 1, 5, and 25 times that of the clinical dose, respectively. The i.v. method of hUC-MSC injection was designed to mimic the clinical administration of hUC-MSCs in humans. According to the principle that the medicinal usage cycle for a long-term toxicity test should be 2–3 times that of clinical medicine usage, we administered hUC-MSCs for 12 weeks. The subjects were observed for 2 weeks after stopping the i.v. injections of hUC-MSCs. In this observation period, all groups of mice displayed normal characteristics, including behavior, appearance, excretion, and lack of death. Animals undergoing i.v. hUC-MSC injections, survived without any hematological or blood biochemistry toxic effects. The necropsy and histopathologic examination of organs also showed no noticeable changes. After the medication was no longer administered for 2 weeks, the above-mentioned examination index still kept in the normal range and no significant differences were observed among the hUC-MSC-treated and control groups. The above results combined with the allergic and oncogenic studies of hUC-MSCs, indicated that i.v. injections of hUC-MSCs with the recommended dosage are safe.
hUC-MSCs was transplanted subcutaneously into Balb/c immunodeficient nude mice, the amount of cells was calculated according to kg body weight, increasing the number of stem cell transplants increases the risk of tumorigenesis (Tung and Knoepfler 2015). However, no abnormality was found by anatomical and histopathological examination after 8 weeks. Through the study of its tumorigenicity in nude mice, the results showed that hUC-MSCs did not have tumorigenicity after short-term subculture, which was consistent with the reports in the literature (Yang et al. 2020).
The properties of MSCs, especially trans-system and trans-embryonic layer differentiation abilities, make these cells potentially ideal candidates for tissue engineering. MSCs are immunophenotypically characterized by the expression of a panel of markers that includes CD73, CD90, CD105, CD44, and HLA class (ABC), whereas they lack expression of hematopoietic stem cell markers CD34, CD45, and CD31 (Sahoo et al. 2017; Pereira et al. 2018). MSCs also express the primitive stem cell markers such as Oct-4, HLA-I, LIF receptor signaling, SSEA-I, and telomerase reverse transcriptase (Conti et al. 2018). MSCs do not provoke a proliferative peripheral blood mononuclear cell response in allogeneic mixed lymphocyte reaction (MLR), thus suggesting a special immunogenic and immunosuppressive role for MSCs (Langroudi et al. 2015; Mohammadpour et al. 2015; Remacha et al. 2015). Furthermore, MSCs can still retain their immunomodulatory function and evade allogeneic rejection after differentiating into other types of cells. The immunodeficient characteristics clearly make MSCs a promising source of stem cells for allogeneic transplantation without the need for a strict major histocompatibility antigen (MHC) match.
hUC-MSCs represent an ideal stem cell source for cell therapies because of their abundant source, convenient collection, and easy isolation, culture, and amplification methods. hUC-MSCs present no trauma or adverse reactions in puerperal and neonates. Furthermore, hUC-MSCs are more primitive cells, they divide rapidly without differentiation, and are free of both ethical concerns and spreading of tumor cells, viruses, and other causative organisms. In the future, hUC-MSCs will present great potential to be widely used in the treatment of clinical diseases, suboptimal health, and prevention of caducity, playing a vital role in human health and improvement of life quality.
Acknowledgements
This work was supported by the study supported by National natural science foundation of China [NO. 81860089] ; Doctoral Foundation of Guizhou Medical University[2020]018, The Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences [2018PT31048] ; Science and Technology Foundation Project of Guizhou Health Committee [gzwjkj2019-1-099].
Author contributions
JX: Conceptualization,Methodology,Software,Investigation,Writing- Original Draft. GL: Methodology, Investigation, Writing—Original Draft. XW: Validation, Formal analysis, Visualization, Software. YH: Validation, Formal analysis, Visualization. HL: Conceptualization, Methodology. LY: Software, Investigation. ZF: Methodology, Software, Investigation. CL: Validation, Formal analysis, Visualization. MK: Visualization, Software. LZ: Methodology, Software, Investigation. YZ: Writing—Review & Editing, Supervision, Data Curation. XQ: Resources, Writing—Review & Editing, Supervision, Data Curation.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request. The datasets generated during or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable' for that section.
Declarations
Conflict of interest
The authors declare no potential conflicts of interest,All authors state that they have nothing to disclose.
Consent to participate
Not applicable' for that section.
Consent for publication
Not applicable' for that section.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianwei Xu and Gang Liu were co-first author and contributed equally to this work.
Contributor Information
Yixia Zhou, Email: 187130703@qq.com.
Xiaolan Qi, Email: 154981793@qq.com.
References
- Bae YK, Kwon JH, Kim M, Kim GH, Choi SJ, Oh W, et al. Intracellular calcium determines the adipogenic differentiation potential of human umbilical cord blood-derived mesenchymal stem cells via the Wnt5a/β-catenin signaling pathway. Stem Cells Int. 2018;2018:6545071. doi: 10.1155/2018/6545071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti G, Bertossi D, Dai Prè E, Cavallini C, Scupoli MT, Ricciardi G, Parnigotto P, Saban Y, Sbarbati A, Nocini P. Regenerative potential of the Bichat fat pad determined by the quantification of multilineage differentiating stress enduring cells. Eur J Histochem. 2018;62:2900. doi: 10.4081/ejh.2018.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Real A, Pérez-Campo FM, Fernández AF, Sañudo C, Ibarbia CG, Pérez-Núñez MI, et al. Differential analysis of genome-wide methylation and gene expression in mesenchymal stem cells of patients with fractures and osteoarthritis. Epigenetics. 2017;12:113–122. doi: 10.1080/15592294.2016.1271854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devito L, Klontzas ME, Cvoro A, Galleu A, Simon M, Hobbs C, et al. Comparison of human isogeneic Wharton's jelly MSCs and iPSC-derived MSCs reveals differentiation-dependent metabolic responses to IFNG stimulation. Cell Death Dis. 2019;10:277. doi: 10.1038/s41419-019-1498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Feng X, Liu J, Xu Y, Pan Q, Ling Z, et al. Characteristics of intestinal microecology during mesenchymal stem cell-based therapy for mouse acute liver Injury. Stem Cells Int. 2019;2019:2403793. doi: 10.1155/2019/2403793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert G, Willemin AS, Jouan-Hureaux V, Louis H, Hupont S, Gillet P, et al. Evaluation of the pro-angiogenic effect of nanoscale extracellular vesicles derived from human umbilical cord mesenchymal stem cells. Bio-Med Mater Eng. 2017;28:S75–S79. doi: 10.3233/BME-171626. [DOI] [PubMed] [Google Scholar]
- Fazeli Z, Abedindo A, Omrani MD, Ghaderian S. Mesenchymal stem cells (MSCs) therapy for recovery of fertility: a systematic review. Stem Cell Rev Rep. 2018;14:1–12. doi: 10.1007/s12015-017-9765-x. [DOI] [PubMed] [Google Scholar]
- Gómez-Aristizábal A, Sharma A, Bakooshli MA, Kapoor M, Gilbert PM, Viswanathan S, Gandhi R. Stage-specific differences in secretory profile of mesenchymal stromal cells (MSCs) subjected to early- vs late-stage OA synovial fluid. Osteoarthritis Cartilage. 2017;25:737–741. doi: 10.1016/j.joca.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Hakkola J, Bernasconi C, Coecke S, Richert L, Andersson TB, Pelkonen O. Cytochrome P450 induction and xeno-sensing receptors pregnane X receptor, constitutive androstane receptor, aryl hydrocarbon receptor and peroxisome proliferator-activated receptor α at the crossroads of toxicokinetics and toxicodynamics. Basic Clin Pharmacol Toxicol. 2018;123:42–50. doi: 10.1111/bcpt.13004. [DOI] [PubMed] [Google Scholar]
- Han B, Zhou L, Guan Q, da Roza G, Wang H, Du C. In vitro expansion and characterization of mesenchymal stromal cells from peritoneal dialysis effluent in a human protein medium. Stem Cells Int. 2018;2018:5868745. doi: 10.1155/2018/5868745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed Pharmacother: Biomed Pharmacother. 2019;109:2318–2326. doi: 10.1016/j.biopha.2018.11.099. [DOI] [PubMed] [Google Scholar]
- Iaffaldano L, Nardelli C, D'Alessio F, D'Argenio V, Nunziato M, Mauriello L, et al. Altered bioenergetic profile in umbilical cord and amniotic mesenchymal stem cells from newborns of obese women. Stem Cells Dev. 2018;27:199–206. doi: 10.1089/scd.2017.0198. [DOI] [PubMed] [Google Scholar]
- Jia L, Gu W, Zhang Y, Ji Y, Liang J, Wen Y, Xu X. The crosstalk between HDPSCs and HUCMSCs on proliferation and osteogenic genes expression in coculture system. Int J Med Sci. 2017;14:1118–1129. doi: 10.7150/ijms.19814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianwei XU, Zhixu HE, Shu L, Wang F et al (2014) Allergy test of human umbilical cord mesenchymal stem cells. J Guiyang Med College 39:5–7+17 (China)
- Jianwei XU, Zhixu HE, Shu L, Wang F (2015) Tumorigenicity test of human umbilical cord mesenchymal stem cells. J Guiyang Med College 40:17–19+23 (China)
- Langroudi L, Hassan ZM, Soleimani M, Hashemi SM. Tumor associated mesenchymal stromal cells show higher immunosuppressive and angiogenic properties compared to adipose derived MSCs. Iran J Immunol. 2015;12:226–239. [PubMed] [Google Scholar]
- Li QC, Liang Y, Su ZB. Prophylactic treatment with MSC-derived exosomes attenuates traumatic acute lung injury in rats. Am J Physiol: Lung Cell Mol Physiol. 2019;316:L1107–L1117. doi: 10.1152/ajplung.00391.2018. [DOI] [PubMed] [Google Scholar]
- Lin T, Pajarinen J, Kohno Y, Huang JF, Maruyama M, Romero-Lopez M, et al. Trained murine mesenchymal stem cells have anti-inflammatory effect on macrophages, but defective regulation on T-cell proliferation. FASEB J. 2019;33:4203–4211. doi: 10.1096/fj.201801845R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadpour H, Pourfathollah AA, Zarif MN, Tahoori MT. TNF-α modulates the immunosuppressive effects of MSCs on dendritic cells and T cells. Int Immunopharmacol. 2015;28:1009–1017. doi: 10.1016/j.intimp.2015.07.045. [DOI] [PubMed] [Google Scholar]
- Mussano F, Genova T, Petrillo S, Roato I, Ferracini R, Munaron L. Osteogenic differentiation modulates the cytokine, chemokine, and growth factor profile of ASCs and SHED. Int J Mol Sci. 2018;19:1454. doi: 10.3390/ijms19051454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Pinhatti VR, Silveira M, Matzenbacher CA, Freitas T, Silva JD, et al. Isolation and characterization of mesenchymal stem/stromal cells from Ctenomys minutus. Genet Mol Biol. 2018;41:870–877. doi: 10.1590/1678-4685-GMB-2018-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phonchai R, Phermthai T, Kitiyanant N, Suwanjang W, Kotchabhakdi N, Chetsawang B. Potential effects and molecular mechanisms of melatonin on the dopaminergic neuronal differentiation of human amniotic fluid mesenchymal stem cells. Neurochem Int. 2019;124:82–93. doi: 10.1016/j.neuint.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Poon Z, Dighe N, Venkatesan SS, Cheung A, Fan X, Bari S, et al. Correction: bone marrow MSCs in MDS: contribution towards dysfunctional hematopoiesis and potential targets for disease response to hypomethylating therapy. Leukemia. 2019;33:1542. doi: 10.1038/s41375-019-0406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacha AR, Barrachina L, Álvarez-Arguedas S, Ranera B, Romero A, Vázquez FJ, et al. Expression of genes involved in immune response and in vitro immunosuppressive effect of equine MSCs. Vet Immunol Immunopathol. 2015;165:107–118. doi: 10.1016/j.vetimm.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm. 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo AK, Das JK, Nayak S. Isolation, culture, characterization, and osteogenic differentiation of canine endometrial mesenchymal stem cell. Vet World. 2017;10:1533–1541. doi: 10.14202/vetworld.2017.1533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung PY, Knoepfler PS. Epigenetic mechanisms of tumorigenicity manifesting in stem cells. Oncogene. 2015;34:2288–2296. doi: 10.1038/onc.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveden-Nyborg P, Bergmann TK, Lykkesfeldt J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol. 2018;123:233–235. doi: 10.1111/bcpt.13059. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cao TT, Tian ZM, Gao H, Rong LM. Subarachnoid transplantation of human umbilical cord mesenchymal stem cell in rodent model with subacute incomplete spinal cord injury: preclinical safety and efficacy study. Exp Cell Res. 2020;395:112184. doi: 10.1016/j.yexcr.2020.112184. [DOI] [PubMed] [Google Scholar]
- Yousefi B, Sanooghi D, Faghihi F, Joghataei MT, Latifi N. Evaluation of motor neuron differentiation potential of human umbilical cord blood- derived mesenchymal stem cells, in vitro. J Chem Neuroanat. 2017;81:18–26. doi: 10.1016/j.jchemneu.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Zhang LB, He M. Effect of mesenchymal stromal (stem) cell (MSC) transplantation in asthmatic animal models: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2019;54:39–52. doi: 10.1016/j.pupt.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wang P, Liu Y, Zhou J, Shi Z, Cheng K, et al. Overexpression of FOXQ1 enhances anti-senescence and migration effects of human umbilical cord mesenchymal stem cells in vitro and in vivo. Cell Tissue Res. 2018;373:379–393. doi: 10.1007/s00441-018-2815-0. [DOI] [PubMed] [Google Scholar]
- Zheng G, Liu Y, Jing Q, Zhang L. Differentiation of human umbilical cord-derived mesenchymal stem cells into hepatocytes in vitro. Bio-Med Mater Eng. 2015;25:145–157. doi: 10.3233/BME-141249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The datasets generated during or analysed during the current study are available from the corresponding author on reasonable request.
Not applicable' for that section.