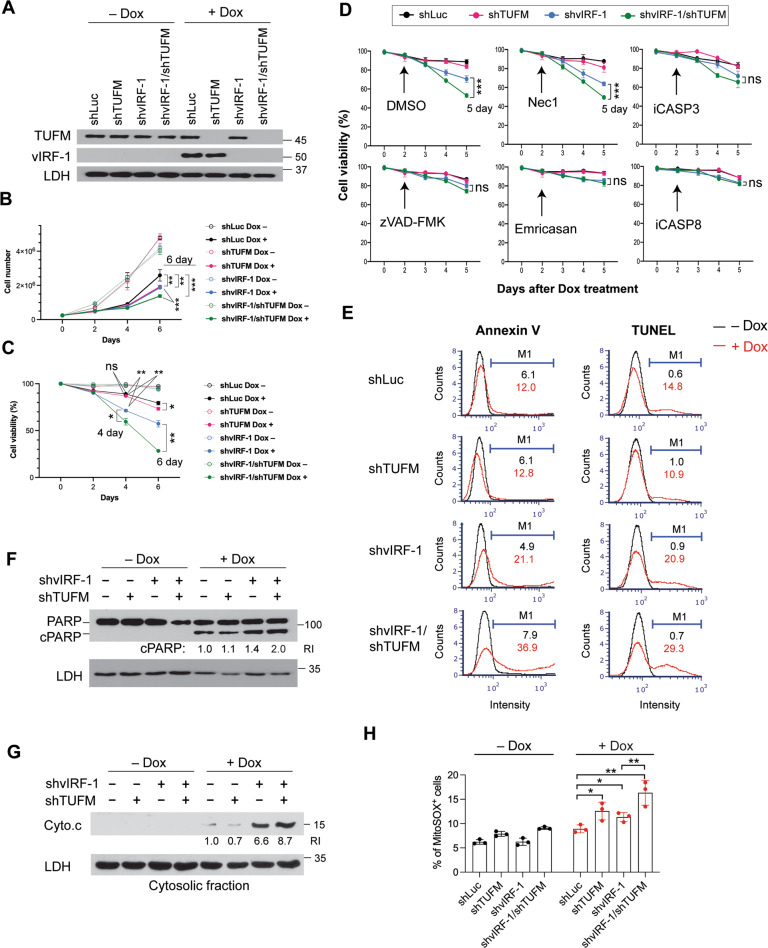
Fig. 2. TUFM depletion potentiates apoptosis induced by vIRF-1 depletion.
A Immunoblots of total-cell extracts derived from iBCBL-1 cells that were stably transduced with the indicated shRNAs and then left untreated or treated with 1 µg/ml Dox for 4 days to induce lytic replication. Lactate dehydrogenase (LDH) was used as a loading control. The iBCBL-1 cell lines were incubated with or without Dox for the indicated days, and cell proliferation and viability were measured using cell counting (B) and trypan blue exclusion (C, D) assays. Necrostatin-1 (Nec-1, 30 µM), the pan-caspase inhibitors zVAD-FMK (20 µM) and Emricasan (10 µM), z-DEVD-FMK (inhibitor of caspase-3, iCASP3, 2 µM), and z-IETD-FMK (inhibitor of caspase-8, iCASP8, 2 µM) were added to the cultures 2 days after Dox treatment (D). Particular datasets (bracketed) were analyzed by t-test for statistical significance. E The iBCBL-1 cell lines were left untreated or treated with Dox for 4 days and analyzed for apoptosis by annexin V staining and terminal deoxynucleotidyl transferase (TdT) dUTP nick end labeling (TUNEL). The proportion of apoptotic cells within each population was determined using an automated cell counter (Nexcelom), and the data were analyzed using FCS Express 6 software. M1 indicates the percentages of apoptotic cells. The total-cell extracts and cytosolic fractions derived from the iBCBL-1 cell lines left untreated or treated with Dox for 3 days were used for immunoblotting analyses of PARP cleavage (F) and cytochrome c release (G). The relative band intensities of cleaved PARP (cPARP) and cytochrome c normalized to LDH are noted under the corresponding bands. H Measurement of mitochondrial superoxide. The iBCBL-1 cell lines left untreated or treated with Dox for 3 days were incubated with 5 µM MitoSOX red reagent in Hank’s balanced salt solution. MitoSOX-positive cells were counted using the automated cell counter. The data present the mean ± SD of triplicate experiments. For B–D, H: *p < 0.05, **p < 0.01, ***p < 0.001, and ns not significant.