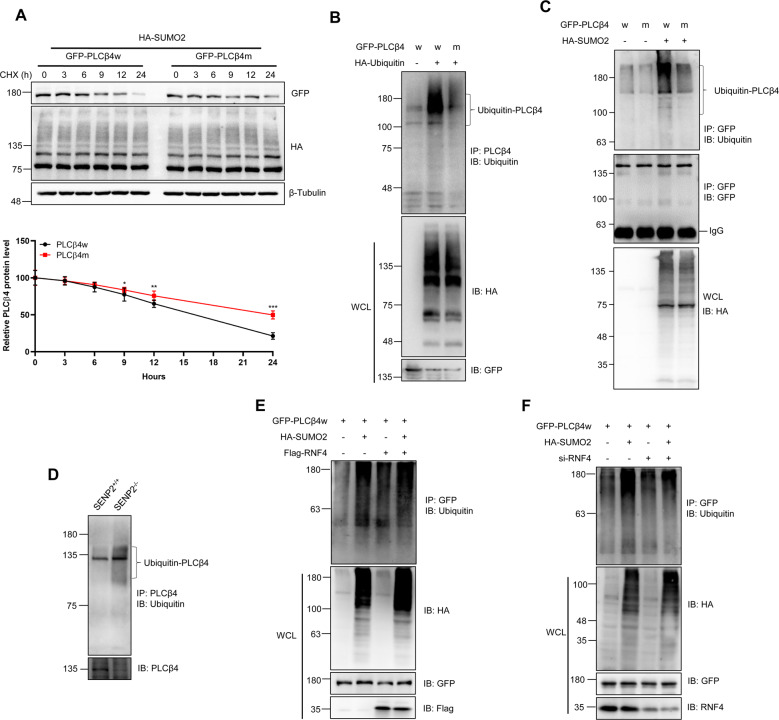
Fig. 3. SUMOylation contributes to the ubiquitination and degradation of PLCβ4 by the STUBL RNF4.
A SUMOylation decreased the stability of the wild-type PLCβ4 but not the mutant PLCβ4. Wild-type PLCβ4 (w) or the triple-SUMO site mutant (m) and HA-SUMO2 were transfected into HEK293T cells, and the cells were treated with CHX for different periods of time. The cells were harvested, and the cell lysates were detected by IB with anti-GFP, anti-HA, or anti-β-Tubulin antibodies (top). The gray analysis was performed by comparing the PLCβ4 band to the β-Tubulin band (bottom, n = 3 repeats/group). B Exogenous ubiquitin induced the ubiquitination of PLCβ4. Wild type or mutant PLCβ4 and HA-ubiquitin plasmids were transfected into HEK293T cells, and the IP with PLCβ4 from cell lysates were detected by IB with anti-Ubiquitin antibody, and the WCL were detected by IB with anti-HA or anti-GFP antibodies. C SUMOylation of PLCβ4 increased its endogenous ubiquitination. PLCβ4w or PLCβ4m and HA-SUMO2 plasmids were transfected into HEK293T cells, and the IP with GFP from cell lysates were detected by IB with anti-ubiquitin antibody, and the WCL were detected by IB with anti-HA or anti-GFP antibodies. D PLCβ4 was modified by endogenous ubiquitin in SENP2−/− MEF cells. The IP with anti-PLCβ4 from MEF cell lysates were detected by IB with anti-ubiquitin antibody. The WCL was detected by IB with anti- PLCβ4 antibody. E RNF4 enhanced the ubiquitination of PLCβ4. The indicated plasmids were transfected into HEK293T cells, and the IP with anti-GFP from cell lysates were detected by IB with anti-ubiquitin antibody. The WCL was detected by IB with anti-HA, anti-GFP, and anti-Flag antibodies. F Ubiquitination of PLCβ4 was decreased after RNF4 knockdown in HEK293T cells. The indicated plasmids were transfected into HEK293T cells, and the IP with anti-GFP from cell lysates were detected by IB with anti-ubiquitin antibody. The WCL was detected by IB with anti-HA, anti-GFP, and anti-RNF4 antibodies.