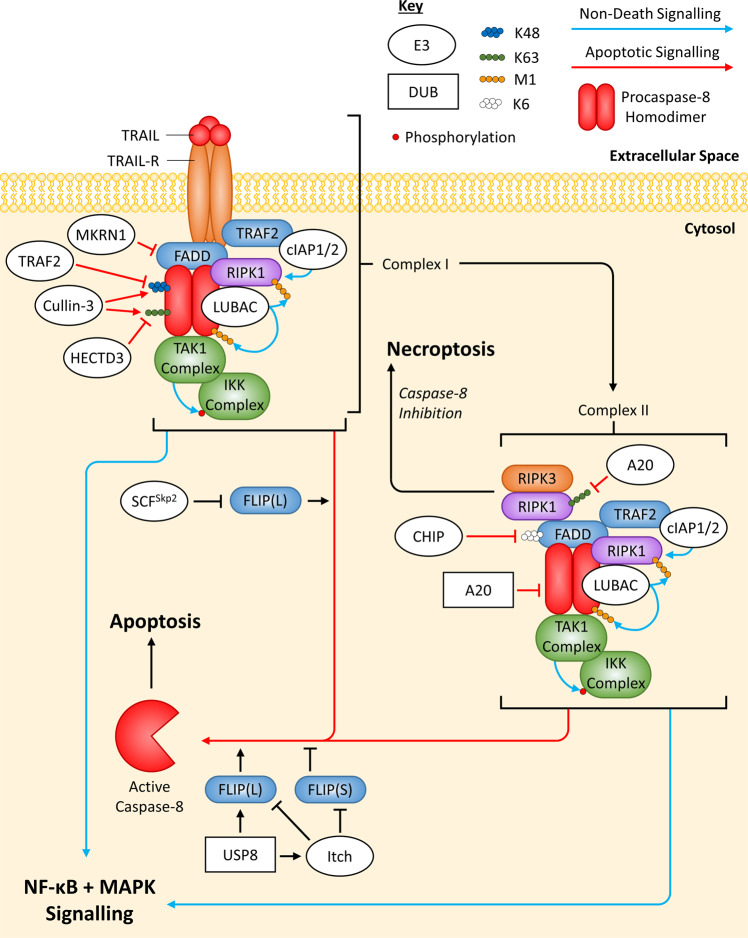
Fig. 4. Overview of ubiquitin-mediated regulation of TRAIL-R apoptotic signalling.
When trimeric TRAIL binds to trimeric TRAIL-R, Complex I is formed through protein:protein interactions and possibly through interactions mediated through Ub chains. Additionally, Complex I is thought to be able to dissociate from TRAIL-R and form Complex II in the cytosol; this has a similar composition to Complex I. Both Complex I and II can activate NF-κB, MAPK and apoptotic signalling, while only Complex II can also activate necroptosis. The exact recruitment process of proteins within Complex I/II is not known; however, Caspase-8’s scaffolding function seems to be important for the interaction of FADD, FLIP(L/S), RIPK1, LUBAC, the TAK1 complex and the IKK complex within Complex I/II, while TRAF2 and cIAP1/2 appear to associate with TRAIL-R. It is not known how TRAF2 and cIAP1/2 associate with Complex II so, for convenience, they have been shown to interact with FADD. E3 ligases/DUBs that regulate Complex I/II do not necessarily regulate each complex specifically, it is for convenience they have been drawn this way. Regulation by E3 ligases (white ovals) and deubiquitinating enzymes DUBs (white rectangles) are indicated. If an E3 ligase/DUB is pointing at a specific Ub chain (indicated by a chain of coloured circles), this means the E3 ligase/DUB is known to regulate the protein by targeting this specific chain; E3 ligases always add Ub chains while DUBs always remove Ub chains. Arrows always indicate positive regulation of a protein they point at (or the protein at the end of the Ub chain they point at), while flat-headed lines indicate the opposite; however, due to FLIP(L)’s controversial signalling, lines pointing from E3 ligases/DUBs to FLIP indicate whether they induce stabilisation (arrows) or degradation (flat-heads) of FLIP. Line colour also indicates the type of signalling that is regulated, either apoptotic (red) or non-death (blue) signalling.