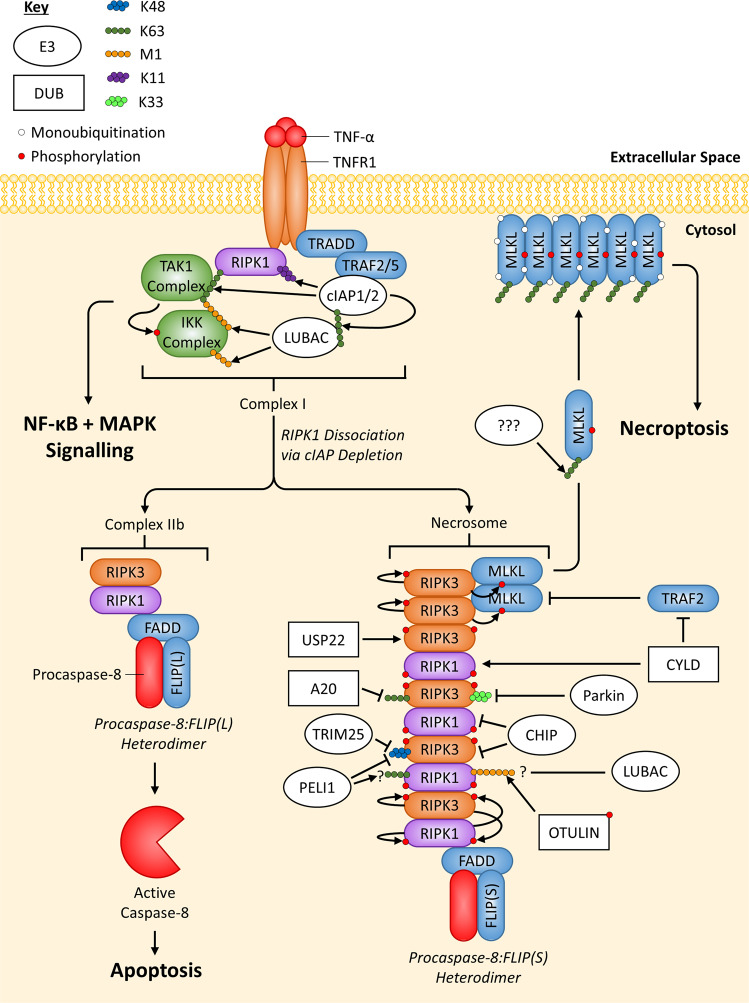
Fig. 5. Overview of ubiquitin-mediated regulation of TNFR1 necroptotic signalling.
When non-ubiquitinated RIPK1 dissociates from TNFR1-induced Complex I, it can form TNFR1-induced Complex IIb. Caspase-8 activity, present in the Procaspase-8:FLIP(L) heterodimer, can negatively regulate RIPK1/3 and inhibit necroptosis, while also regulating apoptosis. However, a lack of Caspase-8 activity within Complex IIb, either from the Procaspase-8:FLIP(S) heterodimer, expression of viral FLIP or treatment with a caspase inhibitor (e.g. zVAD-fmk), leads to phosphorylation and oligomerisation of RIPK1:RIPK3 heterodimers, which can induce the recruitment and phosphorylation of RIPK3 homodimers. RIPK3 homodimers can then recruit and phosphorylate MLKL, which then translocates/oligomerises to the plasma membrane, inducing necroptosis. Complex IIb ubiquitination events have not been highlighted since they are covered in Fig. 3. Regulation by E3 ligases (white ovals) and deubiquitinating enzymes DUBs (white rectangles) are indicated. If an E3 ligase/DUB is pointing at a specific Ub chain (indicated by a chain of coloured circles), this means the E3 ligase/DUB is known to regulate the protein by targeting this specific chain; E3 ligases always add Ub chains while DUBs always remove Ub chains. Arrows always indicate positive regulation of a protein they point at (or the protein at the end of the Ub chain they point at), while flat-headed lines indicate the opposite.