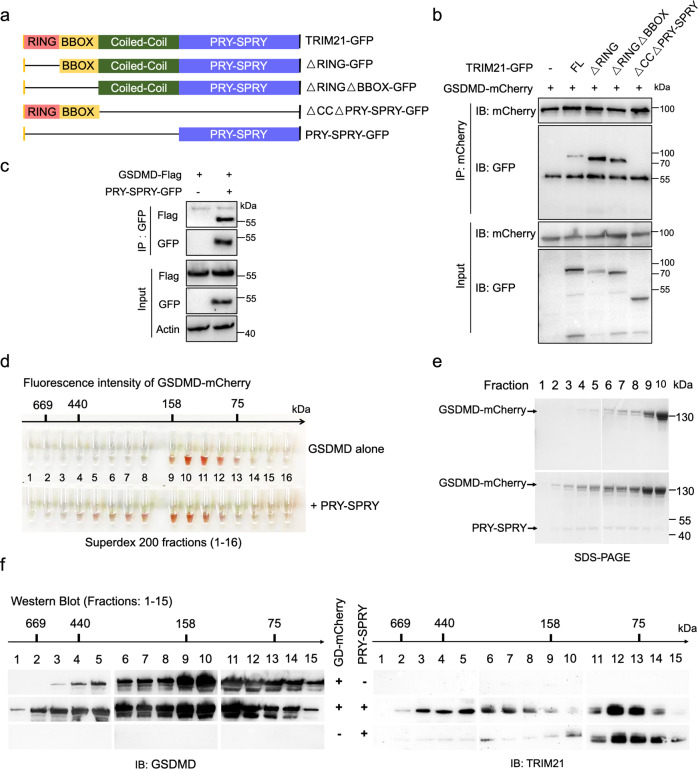
Fig. 2. TRIM21 binds GSDMD via its PRY-SPRY domain.
a The schematic diagram of human TRIM21 and its truncated mutants. b GFP-tagged TRIM21 or its mutants and GSDMD-mCherry were co-transfected into HeLa cells. Cell lysates were immunoprecipitated with an anti-mCherry antibody and then immunoblotted with the indicated antibodies. c The PRY-SPRY domain interacted with GSDMD. PRY-SPRY-GFP and GSDMD-Flag were co-transfected into HEK293T cells. Cell lysates were immunoprecipitated with anti-GFP agarose beads and then immunoblotted with the indicated antibodies. The single-domain antibody used in this assay has no heavy and light chains (KTSM1301, AlpaLife). d–f GSDMD physically binds with the PRY-SPRY domain of TRIM21. The purified His-sumo-GSDMD-mCherry was mixed in a total volume of 500 μL reaction buffer (50 mM Tris-HCl, 150 mM NaCl, 2 mM ATP, 10 mM MgCl2, pH 7.4) in the presence or absence of the His-sumo-PRY-SPRY and incubated for 2 h at 37 °C. His-sumo-GSDMD-mCherry shifted to earlier fractions upon the complex formation with the PRY-SPRY. Fluorescence intensities of eluted fractions were shown in (d). The arrow indicates the fractions of 669-, 440-, 158-, and 75-kDa standard proteins. e SDS-PAGE analysis of the fractions (lanes 1–10) from the analytical gel-filtration column. His-sumo-GSDMD-mCherry shifted to earlier fractions upon the complex formation with the PRY-SPRY. f Western blot analysis of the eluted fractions (1–15) was performed with anti-GSDMD and anti-TRIM21 antibodies. All data are representative of three independent experiments.