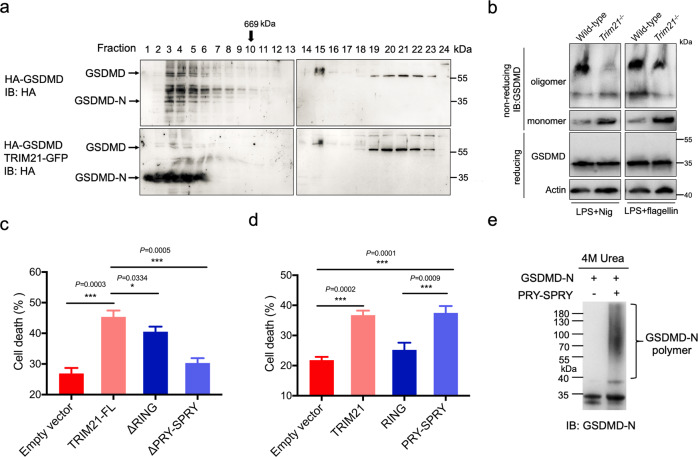
Fig. 6. The PRY-SPRY domain of TRIM21 promotes GSDMD-N oligomerization and cell death.
a TRIM21 promoted GSDMD-N oligomerization. TRIM21−/− HeLa cells were transfected with the indicated constructs. 16 h later, cells were transfected with LPS and treated with Nig. Cells were lysed by the RIPA buffer (containing 1% Triton X-100 and 0.1% SDS) and then lysates were fractionated by gel-filtration chromatography (Superpose 6 column, MW marker at the top) and followed by immunoblotting analysis. b WT and Trim21−/− BMDM cell lines were treated with LPS plus 20 μM Nig or 30 μM Flagellin for 4 h. Cells were lysed in IP lysis buffer and then mixed with loading buffer with or without β-mercaptoethanol and subjected to immunoblotting analysis. c, d TRIM21−/− HeLa cells reconstituted with TRIM21 WT or its mutants were transfected with 1 μg/mL LPS for 4 h followed by 20 μM Nig. Cell death was determined by the CytoTox96 assay. e PRY-SPRY domain of TRIM21 and GSDMD-N were expressed in E. coli BL21 (DE3) cells and further purified by the Ni-affinity chromatography. 5 μg purified GSDMD-N protein (residues 48–265) was mixed in a total volume of 50 μL reaction buffer in the presence or absence of 5 μg His-sumo tagged PRY-SPRY. After incubation at 37 °C for 2 h, reactions were stopped by adding a 5 × loading buffer containing 1% SDS or 4 M urea. GSDMD-N was measured by an anti-cleaved N-terminal GSDMD antibody (Abcam, ab215203). All the data are the means ± SD of triplicate samples from a representative experiment of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.