FIGURE 2.
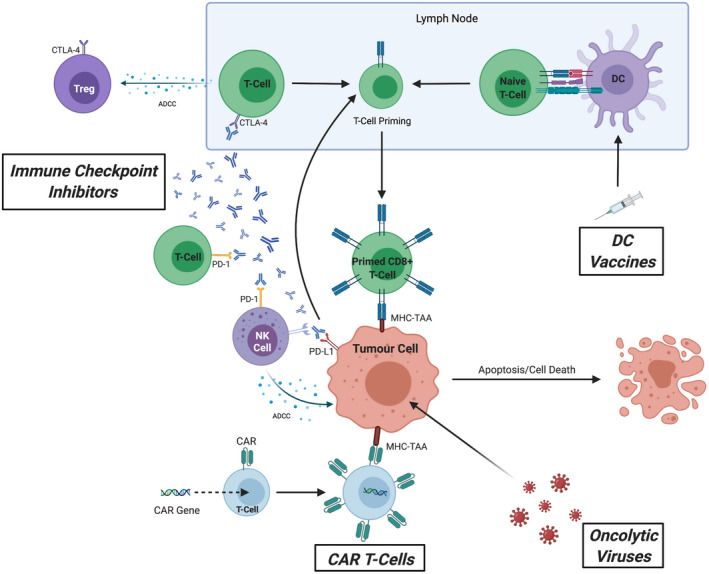
The multifaceted actions of cancer immunotherapy including immune checkpoint inhibitors (ICIs), chimeric antigen receptor (CAR) T‐cells, dendritic cell (DC) vaccines and oncolytic viruses (OVs). Each mode of cancer immunotherapy aims to modulate an individual's immune response against tumour cells, either directly or indirectly, through priming and stimulation to enhance the autologous effect of antitumour activity towards the tumour and inducing tumour apoptosis/cell death or by modifying the surrounding tumour microenvironment to promote immunogenicity. ICIs consist of monoclonal antibodies which target negative immune checkpoint costimulatory molecules expressed on both innate and adaptive immune cells (CTLA‐4, programmed death cell protein 1 [PD‐1], programmed death‐ligand 1 [PD‐L1]) and tumour cells (PD‐L1). These serve to directly inhibit the negative interaction between tumour cells and surrounding host immune cells (through blocking PD‐1/PD‐L1 interaction and CTLA‐4) which upregulates T‐cell priming in the lymph nodes and increases recognition of tumour cells by primed CD8+ T‐cells through major histocompatibility complex (MHC) recognition of tumour‐associated antigens (TAAs) expressed on the surface of tumour cells. Moreover, ICIs also promote antibody‐dependent cellular cytotoxicity (ADCC) against T regulatory (Treg) cells (which primarily serve to downregulate the immune response against tumour cells) and tumour cells themselves through NK cell‐dependent ADCC. CAR T‐cells are exogenously engineered T‐cells expressing a specialised CAR that target specific TAAs to promote a controlled positive downstream immune response. DC vaccines consist of isolated autologous DCs that are primed in vitro against TAAs before being reintroduced into individuals to promote host T‐cell priming. OVs comprise of genetically engineered inactivated viruses that preferentially infect tumour cells and upregulate both humoral and cell‐mediated immune responses 25 , 26 , 27 , 28
