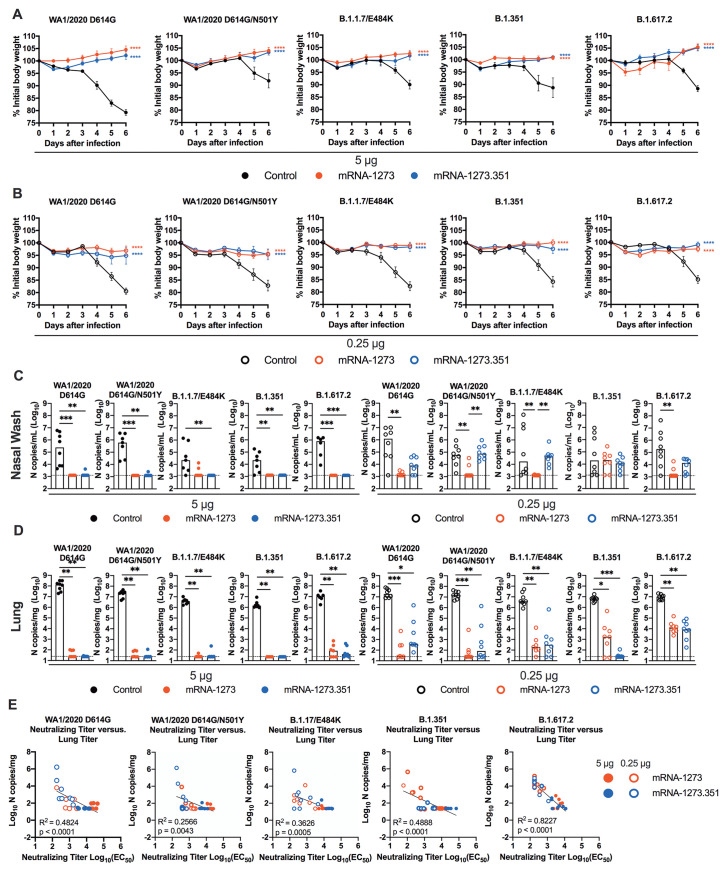
Fig. 5. mRNA vaccination confers protection against SARS-CoV-2 infection in K18-hACE2 transgenic mice.
Seven-week-old female K18-hACE2 mice were immunized and boosted with 5 or 0.25 μg of mRNA vaccines as described in Fig. 4A. Four weeks after boosting, mice were challenged with 103 to 3 × 104 FFU of WA1/2020 D614G, WA1/2020 N501Y/D614G, B.1.1.7/E484K, B.1.351, or B.1.617.2, depending on the strain. (A and B) Body weight change was measured over time. Data are presented as mean ± SEM (n = 8, two experiments). Data were analyzed by a one-way ANOVA of area under the curve from 2 to 4 dpi with Dunnett’s post-test, comparison to control immunized group: ****P < 0.0001. (C and D) Viral burden at 6 dpi in the nasal washes (C) and lungs (D) was assessed by qRT-PCR of the N gene after challenge of immunized mice (n = 6 to 8 mice per group, two experiments). Boxes illustrate median values, and dotted line shows LOD. Data were analyzed by a one-way Kruskal-Wallis ANOVA with Dunn’s post-test, comparison among all immunization groups: *P < 0.05; **P < 0.01; ***P < 0.001). (E) Correlation analyses are shown comparing serum neutralizing antibody concentrations three weeks after boosting plotted against lung viral titer (6 dpi) in K18-hACE2 mice after challenge with the indicated SARS-CoV-2 strain. Pearson’s correlation P and R2 values are indicated as insets. Closed symbols 5 μg vaccine dose; open symbols, 0.25 μg vaccine dose.