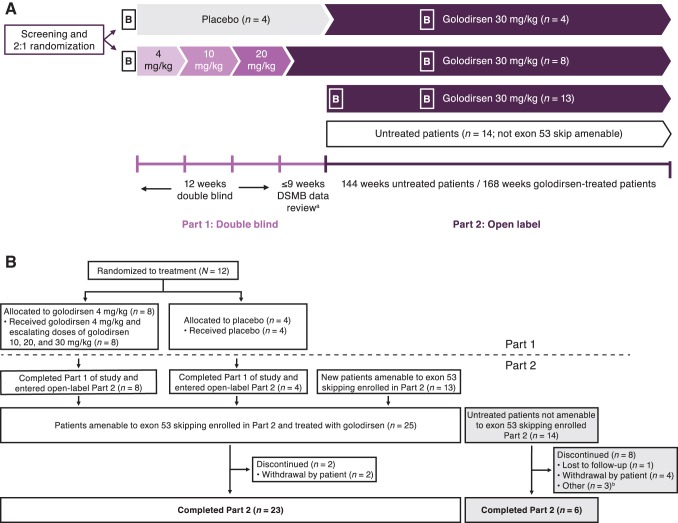
FIG. 1.
(A) Study design. Part 1 was a double-blind, placebo-controlled, dose-titration period; each dose level was administered for ≥2 weeks. Part 2 was an open-label extension period, including all patients from Part 1 plus 13 new patients amenable to exon 53 skipping. The untreated arm consisted of patients not amenable to exon 53 skipping and, per protocol, was not a control group but was included to evaluate DMD natural history and exploratory biomarkers. Adapted with permission from Frank et al. [21]. DOI: https://doi.org/10.1212/WNL.0000000000009233. (B) Patient disposition. aPatients continued on treatment as randomized through enrollment and DSMB review. bReasons included enrollment in a therapeutic study (n = 2) and personal reasons (n = 1). B, biopsy; DMD, Duchenne muscular dystrophy; DSMB, Data Safety Monitoring Board.