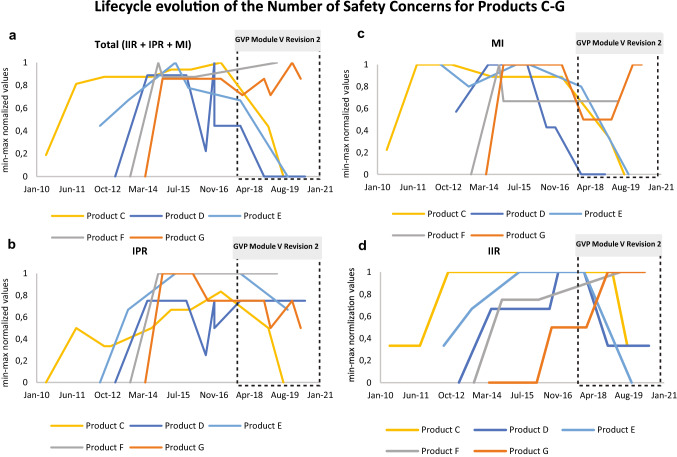
Fig. 3.
Evolution of the number of SCs for five medicinal products in the context of the introduction of GVP Module V Revision 2. The y-axis shows the min-max normalised (0 [min] to 1 [max]) number of product-specific SCs (i.e., IIR, IPR, MI, Total = IIR+IPR+MI) included in the first submitted EU-RMPs and in all subsequently approved EU-RMPs for the indicated medicinal products (Products C, D, E, F and G) between 2010 to 2020. The x-axis shows the period (Month/Year) for the reviewed EU-RMPs date of reports. The black dotted square illustrates the operational period for the GVP Module V Revision 2 Guideline on Risk Management Systems. a Total (IIR + IPR + MI) number of EU-RMP SCs per product over time; b number of EU-RMP IPRs per product over time; c number of EU-RMP MI topics per product over time; d number of EU-RMP IIRs per product over time. EU-RMP European Risk Management Plan, GVP Guidelines on Good Pharmacovigilance Practices, HA health authority, IIR important identified risk, IPR important potential risk, MI missing information, PV pharmacovigilance, RM risk minimisation, SCs safety concerns