Fig. 4.
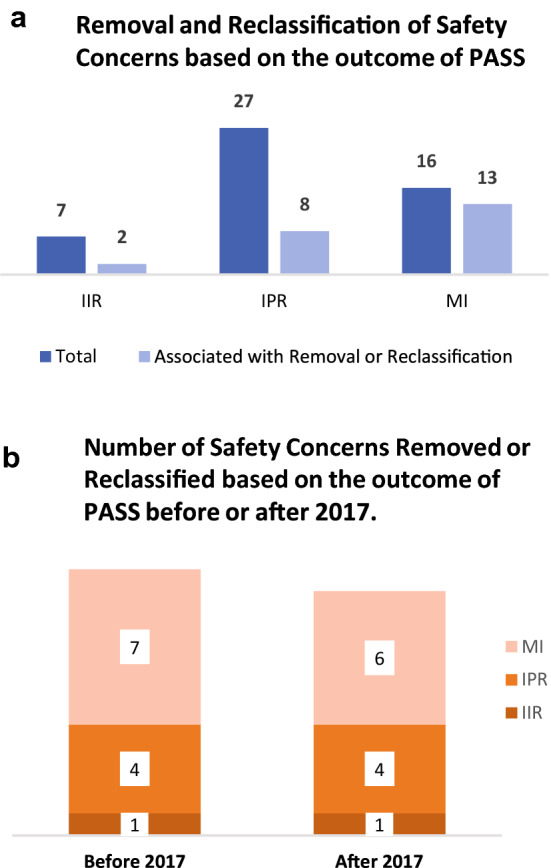
The influence of PASS on the removal and reclassification of SCs. Figure shows the absolute number of European Risk Management Plans (EU-RMPs) SCs (i.e., IIR, IPR and MI) with additional (a) Pharmacovigilance (PV) activities, such as PASS, for Products A, B, C, D, E, F, and G any time point between 2006 and 2020, and the number of SCs (i.e., IIR, IPR and MI) whose removal or reclassification were associated with results from an aPV activity. a The absolute number of SCs that were removed or reclassified based on results from at least one aPV activity compared to the total number of SCs with at least one aPV activity b The absolute number of SCs that were removed or reclassified based on results from completed aPV activities before or after the implementation of GVP Module V Revision 2 in 2017, respectively. aPV additional pharmacovigilance, EU-RMP European Risk Management Plan, GVP Guidelines on Good Pharmacovigilance Practices, HA health authority, IIR important identified risk, IPR important potential risk, MI missing information, PASS Post-authorisation Safety Studies, PV pharmacovigilance, RM risk minimisation, SCs safety concerns
