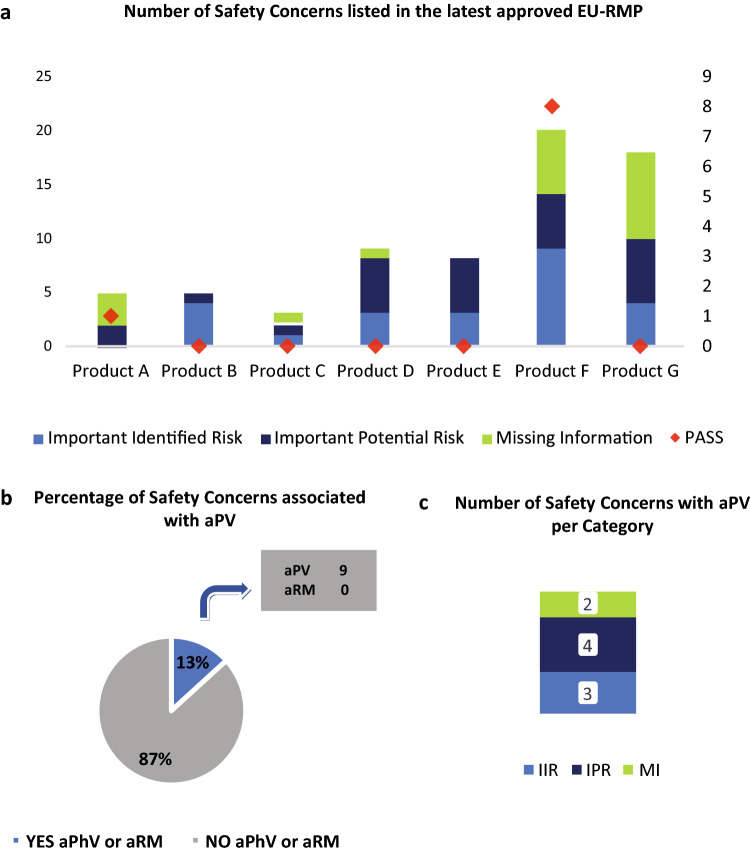
Fig. 5.
EU-RMPs SCs with ongoing or planned aPV activities. Data were obtained from the latest HA-approved EU-RMPs as of 01 October 2020 for each of the seven studied medicinal products. a The left y-axis shows the absolute number of SCs included in the EU-RMP for per indicated medicinal product (x-axis) and the sum of the SCs (i.e., IIR, IPR, MI) for all 7 studied medicinal products. Above each column are dots (right y-axis) representing the total number of SCs per product with ongoing or planned additional (a) PV activities or (a) RM measures (red); b The percentage of the total number of EU-RMP SCs with ongoing or planned aPV activities or aRM measures (YES) versus SCs having no ongoing or planned additional PV or RM activities (NO); c The absolute number of EU-RMP SCs per risk category (i.e., IIR, IPR, MI) with ongoing or planned aPV activities. aPV additional pharmacovigilance, EU-RMP European Risk Management Plan, GVP Guidelines on Good Pharmacovigilance Practices, HA health authority, IIR important identified risk, IPR important potential risk, MI missing information, PASS Post-authorisation Safety Studies, PV pharmacovigilance, RM risk management, SCs safety concerns