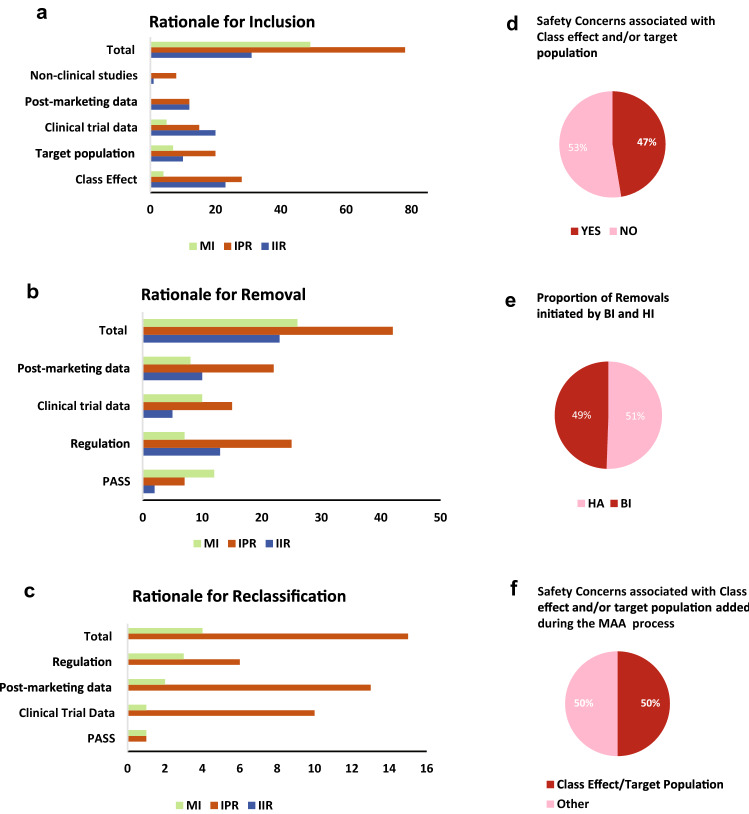
Fig. 6.
Types of sources that resulted in the inclusion, removal or reclassification of SCs listed in EU-RMP. Data on the types of sources (categories defined in Box 3) influencing the lifecycle evolution of SCs were obtained from EU-RMPs for all seven medicinal (excluding the IIRs for Product B). Multiple types of sources could be associated with the inclusion, removal or reclassification of each unique SC. a The x-axis shows the total number of unique SCs included in the reviewed EU-RMPs at any point in time, and the number of SCs whose inclusion in the EU-RMP was associated with evidence from the specified
source category (y-axis); b The x-axis shows the total number of SCs that were removed from the EU-RMPs during the studied period, and the number of SCs whose removal from the EU-RMP was based on data from the indicated source category (y-axis); c The x-axis shows the total number of reclassified safety concerns, and the number of SCs whose reclassification from IPR to IIR or from MI to IPR in the EU-RMP was based on information from the indicated category (y-axis); d The proportion (%) of reclassified SCs associated with (YES) or not with (NO) Pharmacological Class Effect and/or Target Population characteristics; e shows the proportion (%) of removed SCs triggered by a HA versus initiated by BI; f shows the proportion (%) of SCs associated with Pharmacological Class Effect and/or Target Population Characteristics that was added in response to feedback from regulators during the market authorisation application process. aPV additional pharmacovigilance, BI Boehringer Ingelheim, EU-RMP European Risk Management Plan, GVP Guidelines on Good Pharmacovigilance Practices, HA health authority, IIR important identified risk, IPR important potential risk, MI missing information, PASS Post-authorisation Safety Studies, PV pharmacovigilance, RM risk management, SCs safety concerns