Extended Data Fig. 1. Trial schema.
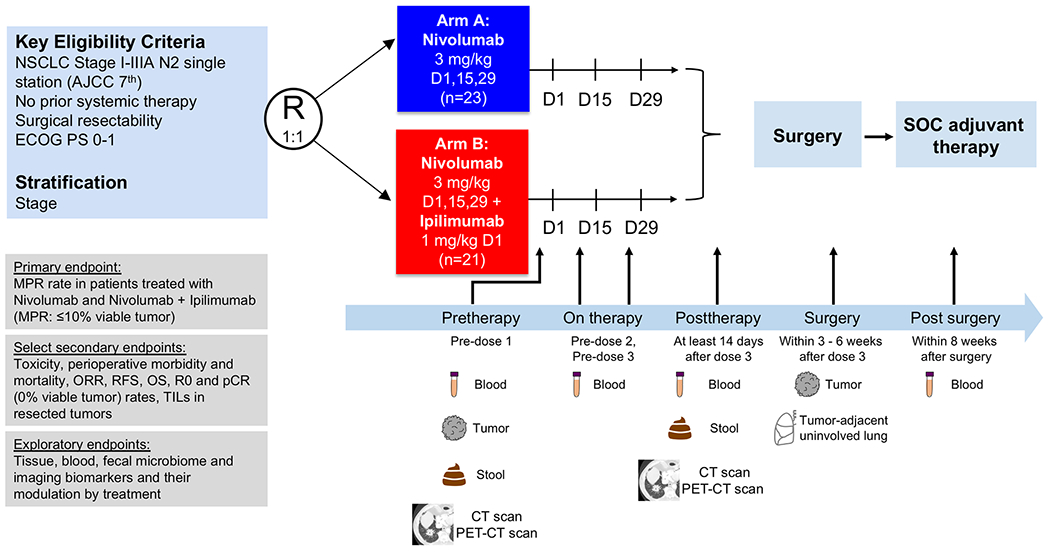
Patients with resectable, pathologically confirmed, clinical stage I-IIIA (N2 single station) NSCLC were stratified by stage and randomized in 1:1 ratio to neoadjuvant nivolumab 3 mg/kg IV every 14 days for up to three doses (arm A; D1, D15 and D29) or ipilimumab 1 mg/kg IV every 6 weeks plus nivolumab 3 mg/kg IV every 14 days for up to three doses (arm B; ipilimumab on D1 only, nivolumab on D1, D15 and D29), followed by surgical resection (at least 3 weeks and within 6 weeks after the last dose of nivolumab). Standard of care adjuvant chemotherapy and/or postoperative radiation therapy were allowed at the discretion of the treating physician. The primary endpoint of the trial was MPR, defined as ≤10% viable tumor in resected tumor specimens. Select secondary endpoints included toxicity, perioperative morbidity and mortality, objective response rates (ORR) by RECIST v.1.1, survival outcomes, radical resection (R0) rate, pathologic complete response (pCR) rate, defined as 0% viable tumor in resected tumor specimens, and quantification of TILs in resected tumor tissues. Select exploratory endpoints included analysis of biomarkers and their modulation by treatment. Imaging studies were performed with CT and PET-CT scans pretherapy (prior to first dose) and at least 14 days after the last dose of neoadjuvant therapy before surgical resection (posttherapy). Tumor samples were collected pretherapy and at surgery together with tumor-adjacent uninvolved lung tissue. Stool samples were collected pretherapy and posttherapy (prior to surgery). Longitudinal blood samples were collected pretherapy, prior to dose 2 and 3, posttherapy (prior to surgery) and within 8 weeks after surgery. NSCLC, non–small cell lung cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; MPR, major pathologic response; ORR, objective response rate; RFS, recurrence-free survival; OS, overall survival; R0, complete surgical resection; pCR, pathologic complete response; TILs: tumor-infiltrating lymphocytes. D: day of therapy. CT: computed tomography, PET-CT: positron emission tomography-computer tomography scan.
