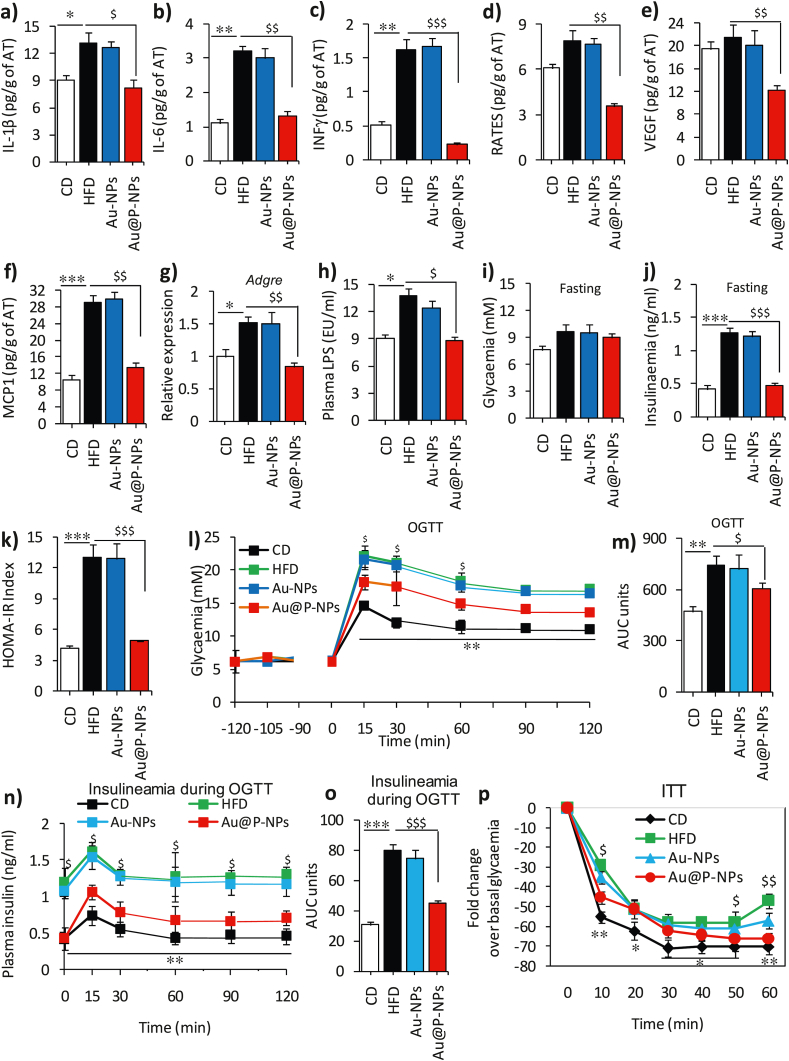
Fig. 4.
Treatment of Au@P-NPs and Au-NPs on inflammation and glucose homeostasis in HFD-fed mice. Protein lysates of eWAT of mice were used to measure (a) IL-1β, (b) IL-6, (c) INF-γ, (d) RANTES, (e) VEGF and (f) MCP-1. (g) Relative mRNA expression of Adgre in eWAT by qPCR. (h) LPS content in blood plasma of 6 h starved animals. Animals were starved from 6 p.m. to 6 a.m. (12 h) at week 6 and subjected to OGTT. NPs were orally given 120 min before to the experiment, and the status of glycaemia was measured 15 min after NPs treatment to examine potential alterations in glycaemia related with sugars present in Au@P-NPs to observe likely alterations in glycaemia linked with sugars of C. verum present in AECV. (i) Glycaemia (fasting) index. (j) Insulinaemia (fasting) index. (k) HOMA-IR. (l) OGTT/glycaemia (nM). (m) OGTT in terms of an area under the curve (AUC). Blood samples were collected during OGTT and employed after glucose administration to measure (n) Insulinaemia during OGTT and (o) Insulinaemia in terms of AUC. Animals were starved for 6 h at week 7 and (p) ITT (insulin tolerance test) was performed after insulin injection at the dose of 1.0 IU/kg through i.p. route. (l, n and p) Two-way frequent measurements ANOVA with BPHT. (a–k, m and o) One-way frequent measurements ANOVA with BPHT. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 for CD against HFD; $P < 0.05, $$P < 0.01 and $$$P < 0.001 for Au@P-NPs against HFD.