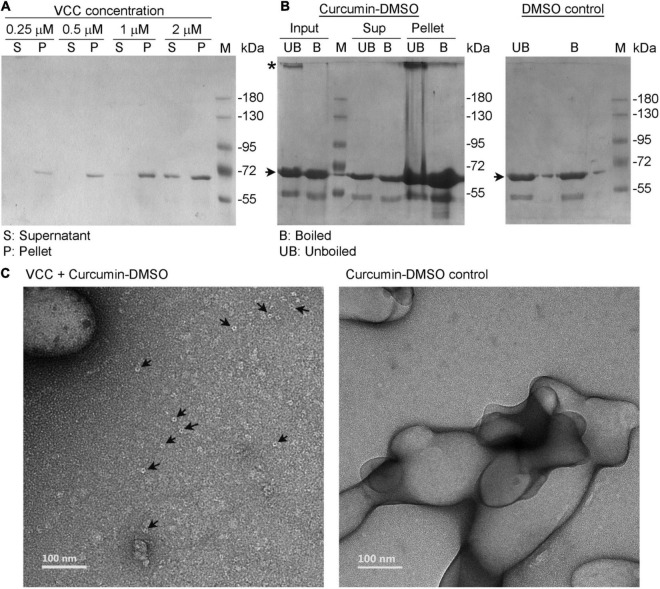
FIGURE 3.
Vibrio cholerae cytolysin tends to associate with the insoluble fraction of curcumin and forms SDS-stable oligomers. (A) Curcumin, from a stock solution prepared in DMSO, was added to VCC (at four different concentrations; 0.25, 0.5, 1, and 2 μM) in the aqueous buffer in PBS. The final concentration of curcumin in the reaction mixture was adjusted to 500 μg/ml. Association of VCC with the insoluble fraction of curcumin, generated upon exposure to the aqueous environment, was probed by the pull down-based assay, in which pellet and the supernatant fractions were analyzed by SDS-PAGE and Coomassie staining. (B) Curcumin, from a stock solution prepared in DMSO, was added to VCC (4 μM) in the aqueous buffer in PBS, and the final concentration of curcumin was adjusted to 500 μg/ml. SDS-stable oligomer formation by VCC associated with the insoluble fraction of curcumin was probed by pull down-based assay, in which the pellet and the supernatant fractions were analyzed by SDS-PAGE and Coomassie staining, with or without boiling in the SDS-PAGE sample buffer. Unboiled fractions allowed detection of the SDS-stable oligomer formation by VCC, if any. For a control reaction, equivalent volume of DMSO, lacking curcumin, was also added to VCC (right panel). The bands corresponding to the monomeric and oligomeric form of VCC are indicated with arrow and asterisk, respectively. Lanes marked as M show the protein molecular weight markers. Data shown here are the representatives of three independent experiments. (C) TEM-based imaging showed formation of some ring-like structure by VCC (indicated with arrow) upon association with the insoluble fraction of curcumin (left panel). Such structures were not documented in the curcumin control without VCC (right panel).