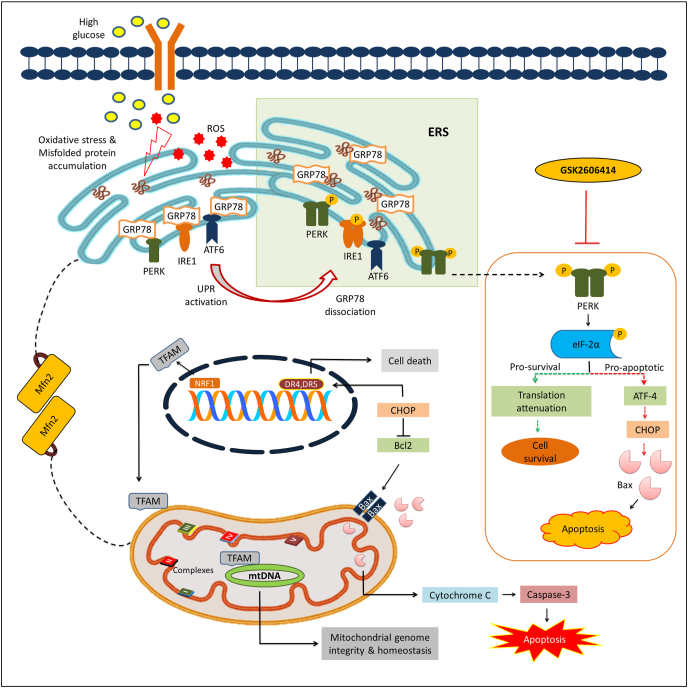
Fig. 6.
Probable mechanistic basis of GSK2606414 against high glucose induced neurotoxicity. High glucose exposure to N2A cells causes oxidative stress and alters protein folding capacity of ER by accumulation of misfolded/unfolded proteins that triggers UPR. UPR is executed by activation of three transmembrane proteins and their respective downstream signalling events by which ERS response exhibits a dichotomic module. PERK plays a critical role in UPR which decides the cell fate where initial mild or acute stress favors the activation of pro-survival module (represented in green dotted line) that results in either amelioration of initial stress or adaptation to it. However, chronic stress induces cell death by activating pro-apoptotic events (represented in red dotted line). Further ERS potentiates mitochondrial dysfunction by multiple mechanisms including excess generation of ROS, CHOP/Bax/Cytochrome C release and altering ER-mitochondria tethering via modulation of Mfn2. GSK2606414, a synthetic PERK inhibitor, modulates the PERK/p-eIF2α/ATF4/CHOP signalling axis and augments mitochondrial function to mitigate high glucose induced neurotoxicity in N2A cells. GRP78: glucose-regulated protein of 78 kDa, ROS: Reactive Oxygen Species, PERK: protein kinase activated by double stranded RNA-like ER kinase, ATF-6: Activating transcription factor 6, ATF-4: Activating transcription factor 4, eIF2α: Eukaryotic Initiation Factor 2 α, IRE1α: Inositol-requiring enzyme 1 alpha, UPR: Unfolded Protein Response, CHOP: C/EBP homologous protein, Mfn2: Mitofusin 2, Bax: BCL2-associated X protein, Bcl2: B-cell lymphoma 2, DR4: Death receptor 4, DR5: Death receptor 5, TFAM: Mitochondrial transcription factor A, NRF1: Nuclear respiratory factor 1, mtDNA: Mitochondrial DNA. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)