Abstract
Store-operated Ca2+ entry (SOCE) is a major mechanism controlling Ca2+ signaling and Ca2+-dependent functions and has been implicated in immunity, cancer, and organ development. SOCE-dependent cytosolic Ca2+ signals are affected by mitochondrial Ca2+ transport through several competing mechanisms. However, how these mechanisms interact in shaping Ca2+ dynamics and regulating Ca2+-dependent functions remains unclear. In a recent issue, Yoast et al. shed light on these questions by defining multiple roles of the mitochondrial Ca2+ uniporter in regulating SOCE, Ca2+ dynamics, transcription, and lymphocyte activation.
Keywords: calcium, CRAC channels, MCU, mitochondria, NFAT, SOCE, lymphocyte
Abbreviations: [Ca2+]cyt, cytosolic Ca2+ concentration; CDI, Ca2+-dependent inactivation; CRAC, Ca2+ release–activated Ca2+; ER, endoplasmic reticulum; IL, interleukin; IP3, inositol-1,4,5-trisphosphate; MCU, mitochondrial Ca2+ uniporter; NFAT, nuclear factor of activated T cells; SOCE, store-operated Ca2+ entry
Calcium signaling regulates many fundamental cell functions, including gene expression, exocytosis, motility, and proliferation. A common mechanism for generating such signals involves Ca2+ release from the endoplasmic reticulum (ER) in response to the generation of the inositol-1,4,5-trisphosphate (IP3) second messenger following cell stimulation by various hormones and growth factors. Depletion of ER Ca2+ triggers the activation of Ca2+ release–activated Ca2+ (CRAC) channels in the plasma membrane allowing Ca2+ influx into the cell (1, 2). This fundamental mechanism known as store-operated Ca2+ entry (SOCE) serves to refill ER Ca2+ stores, shape cytosolic Ca2+ signaling, and regulate numerous Ca2+-dependent functions. Once activated, CRAC channels undergo Ca2+-dependent inactivation (CDI), which limits SOCE through a negative feedback mechanism (1, 2). Mitochondria localized close to the CRAC channels prevent CDI by buffering Ca2+ via the mitochondrial Ca2+ uptake complex (3, 4), which in theory should amplify CRAC-mediated increase in cytosolic Ca2+ concentration ([Ca2+]cyt). However, the net effect of mitochondria on the Ca2+ signal depends on several additional factors, including the ability of mitochondria to release accumulated Ca2+ back into the cytosol, regulate Ca2+ dynamics within the ER, and generate ATP in a Ca2+-dependent manner. The complex problem of how these mitochondria-dependent mechanisms work together to shape SOCE-mediated Ca2+ signaling is the focus of the study by Yoast et al. (5).
Compared with earlier work that relied on pharmacological tools of limited specificity, Yoast et al. (5) employed new molecular tools, which were not available at the time when mitochondria-dependent regulation of CRAC channels was discovered more than 20 years ago (6, 7). They used CRISPR–Cas9 to delete the core molecular component of the mitochondrial Ca2+ uptake complex, mitochondrial Ca2+ uniporter (MCU) (3, 4), in various cell lines from different tissues and species and examined the effects of MCU KO on SOCE-mediated [Ca2+]cyt changes (5). Unexpectedly, and in contrast to the postulated role of mitochondrial Ca2+ buffering in supporting SOCE, they found that MCU KO led to an increase, rather than a decrease, in SOCE-mediated [Ca2+]cyt transients (5). A similar increase was observed in native T and B cells from conditional MCU KO mice.
The authors then systematically examined the effects of MCU KO on other mechanisms contributing to Ca2+ signaling. First, using whole-cell patch-clamp recordings, they found that MCU KO promoted inactivation of CRAC currents, consistent with previous reports that mitochondrial Ca2+ buffering reduces CDI of CRAC channels (6, 7). Second, using subcellular Ca2+ imaging, they showed that MCU KO led to accelerated refilling of ER Ca2+ stores and increased ER Ca2+ content under resting conditions but did not alter activity of the IP3 receptors (5). Third, dissipation of the mitochondrial electrochemical gradient with a protonophore, carbonylcyanide p-trifluoromethoxyphenylhydrazone, blocked SOCE in both WT and MCU KO cells (5), suggesting that the carbonylcyanide p-trifluoromethoxyphenylhydrazone effect was independent of mitochondrial Ca2+ uptake. This finding is particularly insightful because it helps to explain discrepancies between earlier works that relied on the use of protonophores (e.g., carbonyl cyanide m-chlorophenyl hydrazone) and electron transport inhibitors (e.g., antimycin A) to block mitochondrial Ca2+ buffering by inducing mitochondrial depolarization and thereby dissipating the driving force for mitochondrial Ca2+ uptake (6, 8). Although both carbonyl cyanide m-chlorophenyl hydrazone and antimycin A blocked SOCE-mediated [Ca2+]cyt increase in those studies (6, 8), Yoast et al. (5) now clarify that those effects were independent of mitochondrial Ca2+ uptake and likely caused by disrupted mitochondrial respiration.
This study also examined the functional significance of MCU-dependent regulation of SOCE-induced Ca2+ signaling (5). The authors focused on the Ca2+/calcineurin-dependent transcription factor NFAT (nuclear factor of activated T cells) and its control of immune function (9). This choice was well justified, given the central role of SOCE in the regulation of NFAT and NFAT-dependent control of the expression of interleukin 2 (IL-2), IL-4, IL-10, and other cytokines critical for T-cell and B-cell activation and proliferation (1, 2, 10). First, by monitoring SOCE-induced nuclear translocation of NFAT, Yoast et al. showed that MCU KO significantly facilitated activation and nuclear import of NFAT. Second, using conditional MCU KO mice, they found that MCU knockdown specifically in B cells significantly enhanced proliferation of these cells in response to B-cell receptor stimulation. Based on these experiments, the authors concluded that MCU KO/knockdown facilitates NFAT activation and lymphocyte proliferation, consistent with the enhancement of SOCE-driven [Ca2+]cyt elevations in MCU KO cells (5) (Fig. 1). Notably, these findings challenge the conclusion from earlier work that blocking mitochondrial Ca2+ uptake diminishes NFAT activation in immune cells (6). It is important to note that this earlier conclusion was based on the use of a protonophore to block mitochondrial Ca2+ uptake; as now demonstrated by Yoast et al., protonophores block SOCE independent of MCU, explaining the difference between this work (5) and earlier findings (6).
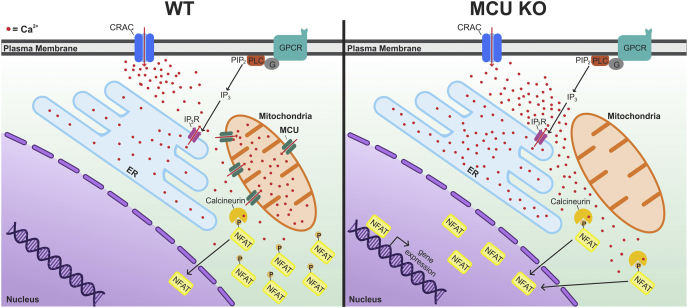
Figure 1.
Summary of the effects of MCU deletion on SOCE-induced cytosolic and organellar Ca2+signaling and activation of the Ca2+-dependent transcription factor NFAT. Activation of G protein–coupled receptors (GPCRs) or tyrosine kinase receptors can initiate phospholipase C (PLC)-dependent synthesis of the lipid messenger IP3 that triggers IP3-receptor (IP3R)-mediated Ca2+ release from the ER. The resulting depletion of the ER Ca2+ stores induces SOCE. This contributes to Ca2+ (red dots) accumulation in the cytosol and activation of the transcription factor NFAT. In WT cells, MCU-mediated Ca2+ uptake by mitochondria reduces CDI of CRAC channels, diminishes refilling of the ER stores with Ca2+ and limits the global cytosolic Ca2+ elevation and activation of NFAT. MCU deletion (MCU KO) reverses all these effects, ultimately resulting in an amplified cytosolic Ca2+ elevation, enhanced NFAT activation, and translocation to the nucleus to initiate a transcription response. Please note that the depiction of the mitochondrial Ca2+ transport has been simplified for clarity. A detailed description of the MCU complex and of mitochondrial Ca2+ efflux systems have been reviewed elsewhere (3, 4). CDI, Ca2+-dependent inactivation; CRAC, Ca2+ release–activated Ca2+; ER, endoplasmic reticulum; IP3, inositol-1,4,5-trisphosphate; MCU, mitochondrial Ca2+ uniporter; NFAT, nuclear factor of activated T cells; SOCE, store-operated Ca2+ entry.
Overall, this study demonstrates that MCU controls multiple aspects of SOCE-mediated Ca2+ signaling, including buffering cytosolic Ca2+, reducing CDI of CRAC channels, and regulating ER Ca2+ store replenishment (Fig. 1). The MCU KO experiments also revealed that the overall contribution of mitochondrial Ca2+ buffering predominates among these multiple competing processes. Despite increased CDI and accelerated Ca2+ extrusion, the net effect of MCU deletion was an increase, rather than a decrease, in SOCE-mediated [Ca2+]cyt transients (Fig. 1). This important conclusion was further supported by extensive mathematical modeling, which systematically tested various contributing factors including CRAC microdomains, CDI, and mitochondrial Na+/Ca2+ exchange.
As with any insightful study that moves the field forward, the article by Yoast et al. outlines new important questions for future research. First, while this work focuses on the role of MCU in shaping cytosolic Ca2+ signals, intramitochondrial Ca2+ regulates many important processes, including ATP synthesis, oxidative stress, and mitochondrial fission. Hence, an important question is how these multiple MCU-dependent functions act in concert to modulate cellular processes triggered by CRAC activation. Second, it is critical to systematically assess the role of MCU–SOCE interaction in regulating other effectors of Ca2+ signaling besides NFAT, including Ca2+-dependent enzymes, other transcription factors, cytoskeletal proteins, and Ca2+ sensors regulating secretion. Finally, unraveling how MCU regulates SOCE and Ca2+-dependent functions in various cell types, including excitable cells (e.g., neurons and muscles) and nonexcitable cells (e.g., platelets, macrophages, and astrocytes), and determining how the cell type–specific molecular composition of MCU complexes and CRAC channels and their subcellular localizations are optimized for the control of distinct functions (i.e., cell migration, muscle contraction, or transmitter release), are important questions to examine. Future research will help address these and many other critical questions inspired by this article.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Funding and additional information
This work was supported by the National Institutes of Health grants (grant nos.: NS096246, DK116624, and NS125884 [to Y. M. U.]). This work was supported by a predoctoral fellowship through the National Institutes of Health T32 grant (grant no.: GM067795 to G. C. W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Edited by Roger Colbran
References
- 1.Prakriya M., Lewis R.S. Store-operated calcium channels. Physiol. Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emrich S.M., Yoast R.E., Trebak M. Physiological functions of CRAC channels. Annu. Rev. Physiol. 2021 doi: 10.1146/annurev-physiol-052521-013426. [DOI] [PubMed] [Google Scholar]
- 3.Kamer K.J., Mootha V.K. The molecular era of the mitochondrial calcium uniporter. Nat. Rev. Mol. Cell Biol. 2015;16:545–553. doi: 10.1038/nrm4039. [DOI] [PubMed] [Google Scholar]
- 4.De Stefani D., Rizzuto R., Pozzan T. Enjoy the trip: Calcium in mitochondria back and forth. Annu. Rev. Biochem. 2016;85:161–192. doi: 10.1146/annurev-biochem-060614-034216. [DOI] [PubMed] [Google Scholar]
- 5.Yoast R.E., Emrich S.M., Zhang X., Xin P., Arige V., Pathak T., Benson J.C., Johnson M.T., Abdelnaby A.E., Lakomski N., Hempel N., Han J.M., Dupont G., Yule D.I., Sneyd J., et al. The mitochondrial Ca(2+) uniporter is a central regulator of interorganellar Ca(2+) transfer and NFAT activation. J. Biol. Chem. 2021;297:101174. doi: 10.1016/j.jbc.2021.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoth M., Button D.C., Lewis R.S. Mitochondrial control of calcium-channel gating: A mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10607–10612. doi: 10.1073/pnas.180143997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilabert J.A., Parekh A.B. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current I-CRAC. EMBO J. 2000;19:6401–6407. doi: 10.1093/emboj/19.23.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoth M., Fanger C.M., Lewis R.S. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J. Cell Biol. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller M.R., Rao A. NFAT, immunity and cancer: A transcription factor comes of age. Nat. Rev. Immunol. 2010;10:645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- 10.Feske S. CRAC channelopathies. Pflugers Arch. 2010;460:417–435. doi: 10.1007/s00424-009-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]