Abstract
Background
Because few studies have epidemiologically evaluated pollen-food allergy syndrome (PFAS), relevant information about this disease is limited in children.
Objective
We wanted to clarify the epidemiological details of PFAS by creating a questionnaire which enables to distinguish class 2 food allergy from that of class 1.
Methods
We conducted a questionnaire survey for schoolchildren attending to public elementary and junior high schools. In this questionnaire, we asked about both the allergy to fruits and/or vegetables and allergic rhinitis (AR). PFAS was, then, defined as allergy for fruits and/or vegetable which occurred after the symptoms of AR appeared.
Results
A total of 2,346 children (median age, 10.6±2.5 years; 1,157 boys) were evaluated. The prevalence of PFAS was 6.9% among subjects. The mean ages in the onset of AR and PFAS were 4.59±2.76 and 7.38±3.17 years old, respectively. Various kinds of foods were shown to be causative, among which kiwifruits were the commonest. As high as approximately 30% of children with PFAS experienced systemic symptoms including cutaneous (21.8%) and respiratory symptoms (9.6%). Anaphylaxis was diagnosed in 5.8% children.
Conclusion
Our results indicated that the prevalence of PFAS was getting higher and the mean age of onset was getting lower. These may be attributed to the increasing number of patients with AR and also to the lower age of onset of AR. We have to be careful to not only local but also systemic symptoms when examining children with PFAS.
Keywords: Anaphylaxis, Fruits-vegetables allergy, Oral allergy syndrome
INTRODUCTION
Food allergy can be classified into class 1 or class 2 based on the route of sensitization. Class 1 is a traditional food allergy in which allergens induce allergic sensitization via the gastrointestinal tract. Class 2 food allergy provokes allergic reactions in patients who have been already sensitized through cross-reactivity with inhaled allergens such as pollen. Pollen-food allergy syndrome (PFAS) is a class 2 food allergy that occurs as a result of cross-reactivity between pollen and fruits or vegetables [1,2,3]. PFAS is an immunoglobulin E-mediated immediate allergic reaction that causes symptoms localized to the oral after patients with pollinosis consume fruits and vegetables. Some cases, however, do exhibit systemic symptoms. Typical types of PFAS are birch pollinosis linked with allergies to Rosaceae fruit (e.g., apples, cherries, and peaches), and poaceous pollinosis linked with allergies to Cucurbitaceae fruits (e.g., watermelons and melons). PFAS is the one of the common food allergies in adults [4]. Considering the recent increase in prevalence and reduction in the age of onset of pollinosis in young Japanese children [5], the high prevalence of PFAS in children and younger age of onset in pediatric PFAS are concerning trends. However, because few studies have epidemiologically evaluated pediatric PFAS, relevant information about this disease is limited. In addition to oral symptoms, PFAS sometimes affects other organs and may cause anaphylactic shock; however, recognition for this disease remains low in the Japanese society, including in schools and among parents, resulting in a lack of appropriate measures for PFAS. In the present study, we performed questionnaires on public elementary and junior high school students in the Northwest area of Saitama prefecture to understand the actual status of PFAS, with the aim of providing them with correct information and care of PFAS and improving their school and family lives.
MATERIALS AND METHODS
Subjects and methods
Public elementary and junior high school students (3,438 children) of Moroyama and Ogose located in the Northwest area of Saitama prefecture were included in this study. This area was located in a suburb surrounded by mountains and forests within commuting distance to the capital. In 2014, we distributed questionnaires to families through the Board of Education of these towns. If young children had difficulty in answering the questionnaire, their parents read each question and completed it based on the children's responses. The survey was conducted between November 2014 and December 2014, and the Board of Education provided the completed questionnaire. Based on the results of the questionnaire, the prevalence of PFAS, age at onset, causative food, and onset symptoms were evaluated. This study was approved by the Institutional Review Board of Saitama Medical University Hospital (approval number: 760-II).
Questionnaires
Children were asked to answer question 1 “Have you experienced any following symptoms after ingesting vegetables or fruits?” as either “yes” or “no.” We prepared a list of induced symptoms as follows: oral tingling, edema in mouth, oral pruritus, edema of lips, edema around mouth itchy throat, sore throat, edema in the ears, itchy ears, tinnitus, rhinorrhea, sneezing, nasal congestion, pruritus, conjunctival erythema, periorbital edema, lacrimation, hoarseness, wheezing, short of breath, cough chest tightness, flushing and pruritus, urticaria, nausea, vomiting, abdominal cramping, diarrhea sense of impending doom, consciousness, lightheadedness, and palpitations. If the answer was “yes,” for 1, children answered age of onset by chronological age in question 2. Furthermore, in question 3, the children were instructed to select the combination of induced symptoms in question 1 and causative foods from the prepared lists. If there were no options, we asked them to fill in the blanks with the relevant combinations. The self-assessment of Allergic Rhinitis and Asthma (SACRA) [6] questionnaire was used to evaluate allergic rhinitis. SACRA was developed based on the guidelines of the Allergic Rhinitis and its Impact on Asthma [7] and the Global Initiative for Asthma [8], and it has been regarded as a highly reliable tool for screening patients with AR and for assessing AR classification [9,10]. A question included in SACRA is “Have you experienced the following symptoms for 1 hour or longer almost every day (if seasonal symptoms, almost every day during the problematic season)?” For this question, the children were asked to choose “yes” or “no” for the following symptoms: (1) watery runny nose; (2) severe and persisted sneezing; (3) stuffy nose (difficulty in nose breathing); (4) itchy nose; and (5) watery eyes. In terms of the age of onset, children were instructed to answer by chronological age in question 5.
Definitions of terms
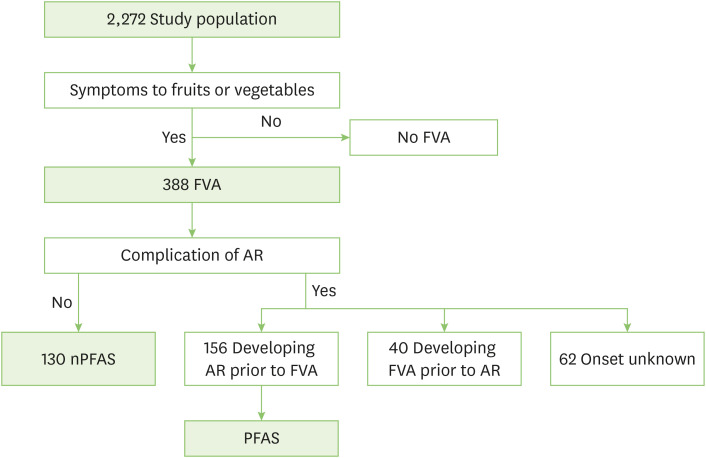
Children who answered “yes” for question 1 (“Have you experienced any symptoms after ingesting vegetables or fruits?”) were defined as having fruits-vegetables allergy (FVA). Children who selected “presence” for one or more items of the 5 questions of the SACRA questionnaire were defined as having AR. Among those in the FVA group, children who developed AR preceding the onset of FVA were regarded as having PFAS (Fig. 1). Among the patients in the FVA group, those without AR were defined as having non-PFAS (nPFAS). Regarding induced symptoms, local symptoms included oral, throat, ocular, nasal, and ear symptoms. Systemic symptoms included cutaneous, respiratory, gastrointestinal, neurological, and cardiovascular symptoms. Children with one or more systemic symptoms were classified into the systemic symptom group, whereas those without systemic symptoms were classified into the local symptom group. According to the guidelines, anaphylaxis was defined as the condition that meets any of the following criteria: (1) Sudden onset of cutaneous symptoms and at least one of the respiratory, gastrointestinal, and cardiovascular symptoms; (2) Two or more of cutaneous, respiratory, gastrointestinal, and cardiovascular symptoms; (3) Sudden reduced blood pressure [11].
Fig. 1. Diagnostic algorithm. FVA, fruits vegetable allergy; AR, allergic rhinitis; nPFAS, no pollen-food allergy syndrome; PFAS, pollen-food allergy syndrome.
RESULTS
Study population
A total of 3,438 school children were included in this study, and responses were obtained from 2,346 children (recovery rate: 68.2%). Seventy-four children were excluded because their sex was unknown. Therefore, 2,272 children were examined. The mean age of children who completed the questionnaire was 10.6±2.5 years (6–15 years), and there were 1,157 boys (50.9%) and 1,115 girls (49.1%). The FVA group had 388 children (17.1%), and among them, the PFAS group and nPFAS group had 156 (6.9%) and 130 children (5.7%), respectively. Forty children (1.8%) developed FVA prior to AR. The temporal relationship between the onset of AR and PFAS was unknown in 62 children. The mean age of the PFAS group was 10.8±2.6 years, whereas that of the nPFAS group was 10.7±2.5 years. The PFAS group had 76 boys (48.7%) and 80 girls (51.3%). The nPFAS group had 54 boys (41.5%) and 76 girls (58.5%).
Details of PFAS group
Prevalence and the age of onset
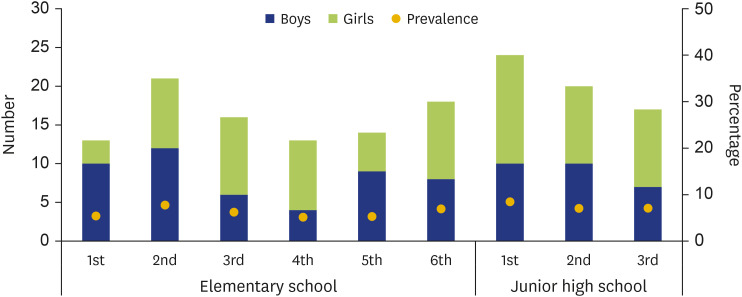
Fig. 2 shows the prevalence and the male-to-female ratio according to academic grades in the PFAS group. The prevalence rate ranged from 5.2% to 8.4%, and the proportion of boys in each grade ranged from 30.8% to 76.9%, with no gender difference. The prevalence rate of PFAS among elementary school students of all grades was 6.1% and that of PFAS among junior high school students of all grades was 8.2% (Fig. 2).
Fig. 2. The number and prevalence of pollen-food allergy syndrome (PFAS) each academic grade in PFAS group. The all number of PFAS was 156 (76 boys and 80 girls). There was no significant difference between boys and girls each academic grade. The mean (±standard deviation) prevalence of PFAS each academic grade was 6.6%±1.2% (range, 5.2%–8.4%). Blue bar and green bar were boys and girls, respectively.
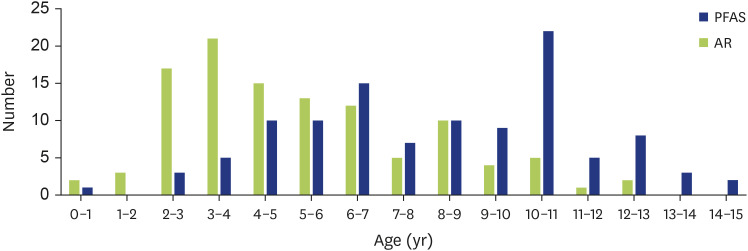
The mean age of AR onset was 4.6±2.8 years, with a peak range from 3 to 4 years. The mean age of PFAS onset was 7.4±3.2 years, which was common from 6 to 7 years and from 10 to 11 years (Fig. 3). The mean period to the onset was 2.8±2.6 years.
Fig. 3. Prevalence and the age of onset in pollen-food allergy syndrome (PFAS) group. The number of children each age in PFAS group. Onset age of AR and PFAS were 4.6±2.8 and 7.4±3.2 years old, respectively (mean±standard deviation). Blue bar was number of PFAS and green bar was number of AR each age. AR, allergic rhinitis.
Causative foods and induced symptoms
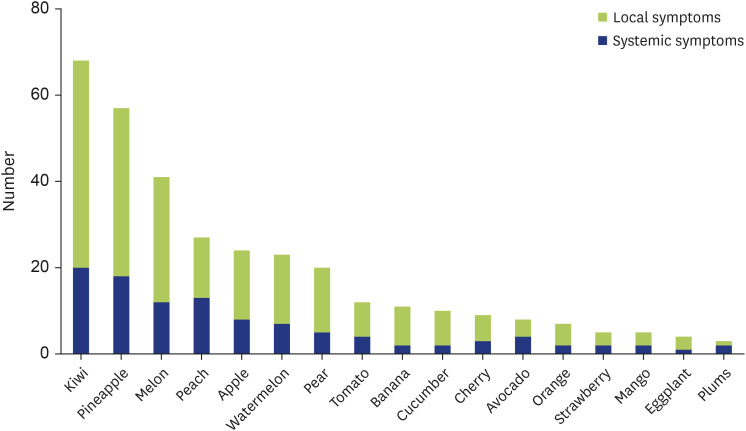
The most common causative foods of 156 children in the PFAS group were kiwifruits in 68 children (43.6%), followed by pineapples in 57 children (36.5%), melons in 41 children (26.3%), apples in 28 children (17.9%), peaches in 27 children (17.3%), watermelons in 24 children (15.4%), pears in 20 children (12.8%), tomatoes in 12 children (7.7%), bananas in 11 children (7.1%), cucumbers in 10 children (6.4%), cherries in 9 children (5.8%), avocados in 8 children (5.1%), oranges in 7 children (4.5%), strawberries in 5 children (3.2%), mangoes in 5 children (3.2%), eggplants in 4 children (2.6%), and plums in 3 children (1.9%). Several other fruits such as apricots were also found to cause symptoms (Fig. 4).
Fig. 4. Causative foods in pollen-food allergy syndrome (PFAS) group. The number of children each causative food in PFAS group. Green and blue bars were local symptoms and systemic symptoms, respectively.
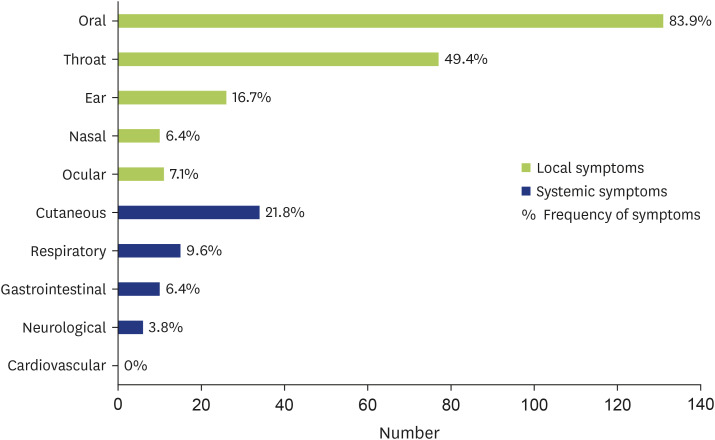
Local and systemic symptoms appeared in 109 (69.9%) and 47 children (30.1%), respectively. Local symptoms, which were described as a cumulative total number of onset symptoms, were oral symptoms in 131 (83.9%), throat symptoms in 77 (49.4%), ear symptoms in 26 (16.7%), nasal symptoms in 10 (6.4%), and ocular symptoms in 11 children (7.1%). Systemic symptoms were cutaneous symptoms in 34 (21.8%), respiratory symptoms in 15 (9.6%), gastrointestinal symptoms in 10 (6.4%), neurological symptoms in 6 (3.8%), and cardiovascular symptoms in 0 children (0.0%) (Fig. 5). A diverse pattern of symptoms was observed in children; specifically, the most common symptoms were pain of the mouth or itchy mouth, itchy throat, rash, and itchy sensation of the skin, followed by itchy eyes or itchy ears, hoarse voice, wheezing, tightness in chest, abdominal pain, and vomiting.
Fig. 5. Numbers and frequency of symptoms in pollen-food allergy syndrome group. Green bar was local symptoms and blue bar was systemic symptoms. Numbers were percentage of symptoms.
The frequency of occurrence of systemic symptoms according to foods was as follows: kiwifruits in 20 of 68 (29.4%), pineapples in 18 of 57 (31.6%), melons in 12 of 41 (29.3%), peaches in 13 of 27 (48.1%), apples in 8 of 28 (28.6%), watermelons in 7 of 24 (29.2%), pears in 5 of 20 (25.0%), tomatoes in 4 of 12 (33.3%), bananas in 2 of 11 (18.2%), cucumbers in 2 of 10 (20.0%), cherries in 3 of 9 (33.3%), avocados in 4 of 8 (50.0%), oranges in 2 of 7 (28.6%), strawberries in 2 of 5 (40.0%), mangoes in 2 of 5 (40.0%), eggplants in 1 of 4 (25.0%), and plums in 2 of 3 children (66.7%) (Fig. 4).
Anaphylaxis was diagnosed in 9 of 156 children (5.8%). The frequency of occurrence of anaphylaxis according to causative foods and other foods was as follows: kiwifruits in 1 of 68 (1.5%), pineapples in 1 of 57 (1.8%), peaches in 2 of 27 (7.4%), melons in 1 of 41 (2.4%), apples in 1 of 24 (4.2%), cherries in 1 of 9 (11.1%), watermelons in 1 of 23 (4.3%), and bananas in 1 of 11 (9.0%).
Comparison between groups with PFAS and that with nPFAS
The number of allergy-causing foods per child was higher in the PFAS group than in the nPFAS group (2.5±2.0 foods vs. 1.9±1.1 foods) (p < 0.05). Local symptoms and systemic symptoms occurred in 109 of 156 children (69.9%) and 47 of 156 children (30.1%), respectively, in the PFAS group, whereas these appeared in 98 of 130 children (75.3%) and 32 of 130 children (24.7%), respectively, in the nPFAS group, with no difference observed between the 2 groups (p = 0.43). The occurrence rate of anaphylaxis was similar between the 2 groups (9 children in the PFAS group [5.7%] and 6 children in the nPFAS group [4.6%]) (p = 0.80) (Table 1).
Table 1. Comparison of between PFAS and nPFAS groups.
| Variable | PFAS (n = 156) | nPFAS (n = 130) | p value | |
|---|---|---|---|---|
| No. of causative foods per person | 2.5±2.0 | 1.9±1.1 | <0.05 | |
| Frequency of symptoms each group | 0.43 | |||
| Local systems | 109 (69.9) | 98 (75.3) | ||
| Systemic symptoms | 47 (30.1) | 32 (24.7) | ||
| Frequency of anaphylaxis | 9 (5.7) | 6 (4.6) | 0.80 | |
Values are presented as mean ± standard deviation or number (%).
PFAS, pollen-food allergy syndrome; nPFAS, no pollen-food allergy syndrome.
T-test were used to compare between the 2 group. Any value of p < 0.05 was considered statistically significant.
DISCUSSION
In this study, by considering the age of onset of AR and FVA, we were able to more accurately investigate PFAS, which is class 2 allergy among fruit and vegetable allergies. Furthermore, this study was not conducted in a medical institution, but in the general population, which helped us understand the characteristics of pediatric PFAS in the general population. Our results suggested that the proportion of FVA that can be classified as class 1 allergy is higher in children than in adults [12,13]. The prevalence of PFAS and the frequency of systemic symptoms were higher [12,14,15] and the mean age of onset of PFAS was lower [16] in our study compared to previous studies. These may be attributed to the increasing number of patients with AR and also to the lower age of onset of AR. However, we cannot simply compare these previous studies with the results of our study due to differences in participant ages.
The concept of FVA includes class 1 and class 2 food allergies. Reportedly, most adults are classified into class 2 [17,18]. However, the present study involving children indicated that 130 children (33.5%) were categorized into the nPFAS group (i.e., absence of AR) among 388 children in the FVA group who developed symptoms after consuming fruits or vegetables. In addition, when adding 40 children who developed FVA preceding AR to the nPFAS group, a maximum of 170 children (45.6%) could be classified into class 1. A previous study showed that approximately half of pediatric FVA were categorized into class 1 food allergy [19], and this trend also was observed in our study. These results suggest that the proportion of FVA associated with class 1 food allergy is higher in children than in adults.
A questionnaire administered to parents of children of elementary and junior high schools showed that the prevalence rate of FVA, including oral allergy syndrome, was 2.4% [12]. In the present study, PFAS was strictly defined after considering the age of onset of AR and FVA based on the general Japanese population; however, the prevalence rate of PFAS in our study area was higher than the previous one (2.4%) [12].
Previous studies showed that the male-to-female ratio of pediatric AR [12,13,20] and that of pediatric PFAS [12,21] are similar or higher in boys than in girls. In the present study, the prevalence rate of pediatric PFAS was similar between boys and girls, thereby confirming previous reports in children.
The present study demonstrated that the onset of PFAS was approximately 3 years later (2.8±2.6 years) than that of AR. Ono et al. [18] reported that many patients developed PFAS within 3 years after the onset of AR in adults, which is consistent with the results of the present study. A study conducted in Italy revealed that the mean age of AR onset was 5.3±2.8 years and that of PFAS onset was 10.5±3.4 years in children aged 4–18 years. In addition, the mean period from pollinosis onset to PFAS onset was 5.2±3.3 years; specifically, 73% of patients developed PFAS within 3 years after pollinosis onset [16]. Our results confirmed previous reports concerning the duration between the onsets of AR and PFAS.
A previous study showed that the prevalence rate of PFAS was higher in individuals who were in their 20s and 30s; however, the incidence of this disease was higher in their teens [22]. A study that evaluated PFAS incidence in different age groups (for a duration of 5 years) showed that the peak of the incidence was found in the age group of 10–14 years, which accounted for 22% of the total [13]. We cannot simply compare the predominant age with previous studies because the age of our subjects was limited (i.e., 6–15 years). Our study indicates that the age of onset may be getting younger.
The most common causative foods for PFAS in the present study were kiwifruits, followed by pineapples, melons, peaches, watermelons, apples, pears, and tomatoes. Several studies have shown that kiwifruits and melons are the most common causative foods for PFAS in many areas of Japan [12,18,19,23]. The number of causative foods per child was significantly higher in the PFAS group than in the nPFAS group. This may be because patients with PFAS respond to panallergens, such as profilin, that show high cross-reactivity with many foods. In previous studies, many patients with PFAS were allergic to 2 or more foods [20,24]. This is consistent with the results of the present study, in which children in the PFAS group were found to be allergic to 2 or more foods on average.
Local symptoms and systemic symptoms were observed in approximately 109 of 156 children (69.9%) and 47 of 156 children (30.1%), respectively, in among children in the PFAS group. The main symptoms of PFAS include oral /pharynx symptoms, which were observed in 75%–95% of patients in the previous studies [4,25,26]. Incidence of oral symptoms was found in 131 of 156 children (83.9%) and pharynx symptoms were observed in 77 children (49.4%). Among systemic symptoms, specifically, skin and respiratory symptoms were prominent (21.8% and 9.6%, respectively).
In a cohort study of PFAS in Southern Europe, 32.3% among patients included at least one systemic symptom [27]. In Korea, 8.7% of PFAS patients had systemic symptoms other than gastrointestinal symptoms, including 43% skin, 20% respiratory, 3.7% cardiovascular, and 4.8% neurological symptoms [14,28]. Previously, the frequency of systemic symptoms ranged from 3% to 5%, whereas in recent years, the frequency seemed to be increasing. In the present study, as high as 30.1% of children had at least one systemic symptom, of which 21.8% had skin and 9.6% had respiratory symptoms. As for the frequency of anaphylaxis, it ranges 1.3 to 8.9% in children [14,15]. In addition, a report from Korea showed that 1.9% of patients experienced anaphylactic shock [14]. In this study, the frequency of anaphylaxis was 5.8%, but no case of anaphylactic shock was observed. It was well known that regional differences were observed in the frequency and emerging symptoms of PFAS. This was because the type of aeroallergen being dispersed was strongly influenced by geographic location and endemic flora [13,15,27,29,30]. Multivariate analysis from Korea indicated that the number of causative foods was a risk factor for the development of anaphylaxis [28]. However, our study did not show a significant correlation between the number of allergens and that of anaphylaxis (data no shown). Following factors might have influenced to the high frequency of systemic symptoms in the present study. First, the respondents who were aware of their food allergies might have been more likely to answer the questionnaires. Second, the definition of anaphylaxis is limited to the combination of relatively severe symptoms of more than 2 organs, and not include those with relative mild ones [11]. In our questionnaire, we asked whether symptoms were present or not, but did not take the severity of symptoms into account. Thus, it was possible that the frequency of anaphylaxis was overestimated in the present study.
Finally, this study had several limitations. First, respondents of the questionnaire were children and thus reliability of their responses may be occasionally low, especially in case of those in lower grades. Second, as this was a questionnaire survey, we were unable to perform the allergen-specific immunoglobulin E measurements and prick test. These factors hindered the objective evaluation of food allergy. However, given that little is known about the relationship between AR onset and food allergy in the general population of PFAS, we believe that our data are helpful for the understanding of recent actual status of PFAS in children.
ACKNOWLEDGEMENTS
The authors thank the Board of Education of Moroyama and Ogose towns for the distribution and collection of questionnaires and the people in public elementary and junior high schools in these towns who participated in this study. This work was supported by Grants-in-Aid for Scientific Research (C) (grant number: 18K07886) from Japan Society for the Promotion of Science.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Takeshi Koga, Kenichi Tokuyama, Atsushi Kamijyo.
- Formal analysis: Takeshi Koga.
- Investigation: Shunichi Ogawa, Eiji Morita, Yutaka Ueda.
- Methodology: Takeshi Koga, Kenichi Tokuyama, Atsushi Kamijyo.
- Project administration: Toshiko Itazawa, Kenichi Tokuyama.
- Writing - original draft: Takeshi Koga.
- Writing - review & editing: Takeshi Koga, Kenichi Tokuyama, Atsushi Kamijyo.
References
- 1.Ma S, Sicherer SH, Nowak-Wegrzyn A. A survey on the management of pollen-food allergy syndrome in allergy practices. J Allergy Clin Immunol. 2003;112:784–788. doi: 10.1016/s0091-6749(03)02008-6. [DOI] [PubMed] [Google Scholar]
- 2.Egger M, Mutschlechner S, Wopfner N, Gadermaier G, Briza P, Ferreira F. Pollen-food syndromes associated with weed pollinosis: an update from the molecular point of view. Allergy. 2006;61:461–476. doi: 10.1111/j.1398-9995.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- 3.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA, Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebisawa M, Ito K, Fujisawa T Committee for Japanese Pediatric Guideline for Food Allergy, The Japanese Society of Pediatric Allergy and Clinical Immunology; Japanese Society of Allergology. Japanese guidelines for food allergy 2020. Allergol Int. 2020;69:370–386. doi: 10.1016/j.alit.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Nishima S, Odajima H, Ota K, Oka N, Okazaki K, Oka M, Hisada N, Kumamoto N, Tatsuo K. A study on the prevalence of allergic disease in school children in western districts of Japan: comparison between the studies in 1992, 2002 and 2012 with the same methods and same districts. Jpn J Pediatr Allergy Clin Immunol. 2013;27:149–169. [Google Scholar]
- 6.Ohta K, Bousquet PJ, Aizawa H, Akiyama K, Adachi M, Ichinose M, Ebisawa M, Tamura G, Nagai A, Nishima S, Fukuda T, Morikawa A, Okamoto Y, Kohno Y, Saito H, Takenaka H, Grouse L, Bousquet J. Prevalence and impact of rhinitis in asthma. SACRA, a cross-sectional nation-wide study in Japan. Allergy. 2011;66:1287–1295. doi: 10.1111/j.1398-9995.2011.02676.x. [DOI] [PubMed] [Google Scholar]
- 7.Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R, Canonica GW, Casale T, Chavannes NH, Correia de Sousa J, Cruz AA, Cuello-Garcia CA, Demoly P, Dykewicz M, Etxeandia-Ikobaltzeta I, Florez ID, Fokkens W, Fonseca J, Hellings PW, Klimek L, Kowalski S, Kuna P, Laisaar KT, Larenas-Linnemann DE, Lødrup Carlsen KC, Manning PJ, Meltzer E, Mullol J, Muraro A, O’Hehir R, Ohta K, Panzner P, Papadopoulos N, Park HS, Passalacqua G, Pawankar R, Price D, Riva JJ, Roldán Y, Ryan D, Sadeghirad B, Samolinski B, Schmid-Grendelmeier P, Sheikh A, Togias A, Valero A, Valiulis A, Valovirta E, Ventresca M, Wallace D, Waserman S, Wickman M, Wiercioch W, Yepes-Nuñez JJ, Zhang L, Zhang Y, Zidarn M, Zuberbier T, Schünemann HJ. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 8.The Global Initiative for Asthma. Revised 2002: Global strategy for asthma management and prevention [Internet] Fontana (WI): The Global Initiative for Asthma; [cited 2021 Dec 22]. Available from: http://www.ginasthma.org/ [Google Scholar]
- 9.Hojo M, Ohta K, Iikura M, Mizutani T, Hirashima J, Sugiyama H. Clinical usefulness of a guideline-based screening tool for the diagnosis of allergic rhinitis in asthmatics: the Self Assessment of Allergic Rhinitis and Asthma questionnaire. Respirology. 2013;18:1016–1021. doi: 10.1111/resp.12116. [DOI] [PubMed] [Google Scholar]
- 10.Yasuo M, Kitaguchi Y, Komatsu Y, Hama M, Koizumi T, Agatsuma T, Ichiyama T, Kato A, Moteki H, Hanaoka M. Self-assessment of Allergic Rhinitis and Asthma (SACRA) Questionnaire-based allergic rhinitis treatment improves asthma control in asthmatic patients with allergic rhinitis. Intern Med. 2017;56:31–39. doi: 10.2169/internalmedicine.56.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simons FE, Ardusso LR, Bilò MB, El-Gamal YM, Ledford DK, Ring J, Sanchez-Borges M, Senna GE, Sheikh A, Thong BY World Allergy Organization. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011;127:587–93.e1-22. doi: 10.1016/j.jaci.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Kato T, Yamamoto K, Watanabe T, Kawabe Y. A surveillance of fruits allergy in school children in Gamagori city Aichi prefecture. Jan J Pediatr Allergy Clin Immunol. 2015;29:676–684. [Google Scholar]
- 13.Skypala IJ, Bull S, Deegan K, Gruffydd-Jones K, Holmes S, Small I, Emery PW, Durham SR. The prevalence of PFS and prevalence and characteristics of reported food allergy; a survey of UK adults aged 18-75 incorporating a validated PFS diagnostic questionnaire. Clin Exp Allergy. 2013;43:928–940. doi: 10.1111/cea.12104. [DOI] [PubMed] [Google Scholar]
- 14.Kim MA, Kim DK, Yang HJ, Yoo Y, Ahn Y, Park HS, Lee HJ, Jeong YY, Kim BS, Bae WY, Jang AS, Park Y, Koh YI, Lee J, Lim DH, Kim JH, Lee SM, Kim YM, Jun YJ, Kim HY, Kim Y, Choi JH. Pollen food allergy syndrome in Korean pollinosis patients: a nationwide survey. Allergy Asthma Immunol Res. 2018;10:648–661. doi: 10.4168/aair.2018.10.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010;104:101–108. doi: 10.1016/j.anai.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Dondi A, Tripodi S, Panetta V, Asero R, Businco AD, Bianchi A, Carlucci A, Ricci G, Bellini F, Maiello N, del Giudice MM, Frediani T, Sodano S, Dello Iacono I, Macrì F, Massaccesi V, Caffarelli C, Rinaldi L, Patria MF, Varin E, Peroni D, Chinellato I, Chini L, Moschese V, Lucarelli S, Bernardini R, Pingitore G, Pelosi U, Tosca M, Paravati F, La Grutta S, Meglio P, Calvani M, Plebani M, Matricardi PM. Pollen-induced allergic rhinitis in 1360 Italian children: comorbidities and determinants of severity. Pediatr Allergy Immunol. 2013;24:742–752. doi: 10.1111/pai.12136. [DOI] [PubMed] [Google Scholar]
- 17.Ikezawa Z. [Oral hypersensitivity syndrome and latex allergy] Nippon Naika Gakkai Zasshi. 2004;93:1032–1040. doi: 10.2169/naika.93.1032. [DOI] [PubMed] [Google Scholar]
- 18.Ono E, Maeda Y, Tanimoto H, Fukutomi Y, Oshikata C, Sekiya K, Tuburai T, Turikisawa N, Otomo M, Taniguchi M, Ishii H, Asahina A, Miyazaki E, Kumamoto T, Akiyama K. Clinical features of oral allergy syndrome to plant foods allergens in Kanto regions. Arerugi. 2007;56:587–592. [PubMed] [Google Scholar]
- 19.Sugii K, Tachimoto H, Syukuya A, Suzuki M, Ebisawa M. Association between childhood oral allergy syndrome and sensitization against four major pollens (Japanese cedar, orchard grass, short ragweed, alder) Arerugi. 2006;55:1400–1408. [PubMed] [Google Scholar]
- 20.Ortolani C, Ispano M, Pastorello E, Bigi A, Ansaloni R. The oral allergy syndrome. Ann Allergy. 1988;61:47–52. [PubMed] [Google Scholar]
- 21.Brown CE, Katelaris CH. The prevalence of the oral allergy syndrome and pollen-food syndrome in an atopic paediatric population in south-west Sydney. J Paediatr Child Health. 2014;50:795–800. doi: 10.1111/jpc.12658. [DOI] [PubMed] [Google Scholar]
- 22.Asakura K, Honma T, Yamazaki N, Ishikawa T. Relationships between oral allergy syndrome and sensitization to pollen antigen, especially to mugwort. Arerugi. 2006;55:1321–1326. [PubMed] [Google Scholar]
- 23.Adachi A, Horikawa T. Epidemiological survey of oral allergy syndrome with pollinosis and food-dependent exercise lnduced anaphylaxis among elementary, junior high and senior high school children in Higashi Harima District of Hyogo. J Environ Dermatol Cutan Allergol. 2007;1:102–108. [Google Scholar]
- 24.Kato T, Tajima N, Kitamura K, Takasato Y, Tajima I, Ono M, Tagami K, Sakai K, Furuta T, Sugiura S, Ito K. Oral and systemic symptoms in children with fruit allergy. Arerugi. 2018;67:129–138. doi: 10.15036/arerugi.67.129. [DOI] [PubMed] [Google Scholar]
- 25.Anderson LB, Jr, Dreyfuss EM, Logan J, Johnstone DE, Glaser J. Melon and banana sensitivity coincident with ragweed pollinosis. J Allergy. 1970;45:310–319. doi: 10.1016/0021-8707(70)90037-7. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson NE, Wihl JA, Arrendal H. Birch pollen-related food hypersensitivity: influence of total and specific IgE levels. A multicenter study. Allergy. 1983;38:353–357. doi: 10.1111/j.1398-9995.1983.tb04130.x. [DOI] [PubMed] [Google Scholar]
- 27.Lipp T, Acar Şahin A, Aggelidis X, Arasi S, Barbalace A, Bourgoin A, Bregu B, Brighetti MA, Caeiro E, Caglayan Sozmen S, Caminiti L, Charpin D, Couto M, Delgado L, Di Rienzo Businco A, Dimier C, Dimou MV, Fonseca JA, Goksel O, Guvensen A, Hernandez D, Hoffmann TM, Jang DT, Kalpaklioglu F, Lame B, Llusar R, Makris M, Mazon A, Mesonjesi E, Nieto A, Öztürk A, Pahus L, Pajno G, Panasiti I, Papadopoulos NG, Pellegrini E, Pelosi S, Pereira AM, Pereira M, Pinar NM, Potapova E, Priftanji A, Psarros F, Sackesen C, Sfika I, Suarez J, Thibaudon M, Travaglini A, Tripodi S, Verdier V, Villella V, Xepapadaki P, Yazici D, Matricardi PM, Dramburg S. Heterogeneity of pollen food allergy syndrome in seven Southern European countries: The @IT.2020 multicenter study. Allergy. 2021;76:3041–3052. doi: 10.1111/all.14742. [DOI] [PubMed] [Google Scholar]
- 28.Kim M, Ahn Y, Yoo Y, Kim DK, Yang HJ, Park HS, Lee HJ, Kim MA, Jeong YY, Kim BS, Bae WY, Jang AS, Park Y, Koh YI, Lee J, Lim DH, Kim JH, Lee SM, Kim YM, Jun YJ, Kim HY, Kim Y, Choi JH. Clinical manifestations and risk factors of anaphylaxis in pollen-food allergy syndrome. Yonsei Med J. 2019;60:960–968. doi: 10.3349/ymj.2019.60.10.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson G, Coop C. Pollen food allergy syndrome (PFAS): a review of current available literature. Ann Allergy Asthma Immunol. 2019;123:359–365. doi: 10.1016/j.anai.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Kiguchi T, Yamamoto-Hanada K, Saito-Abe M, Sato M, Irahara M, Ogita H, Miyagi Y, Inuzuka Y, Toyokuni K, Nishimura K, Ishikawa F, Miyaji Y, Kabashima S, Fukuie T, Narita M, Ohya Y. Pollen-food allergy syndrome and component sensitization in adolescents: a Japanese population-based study. PLoS One. 2021;16:e0249649. doi: 10.1371/journal.pone.0249649. [DOI] [PMC free article] [PubMed] [Google Scholar]