Scheme 1. Synthesis of Intermediates 1 and 2a.
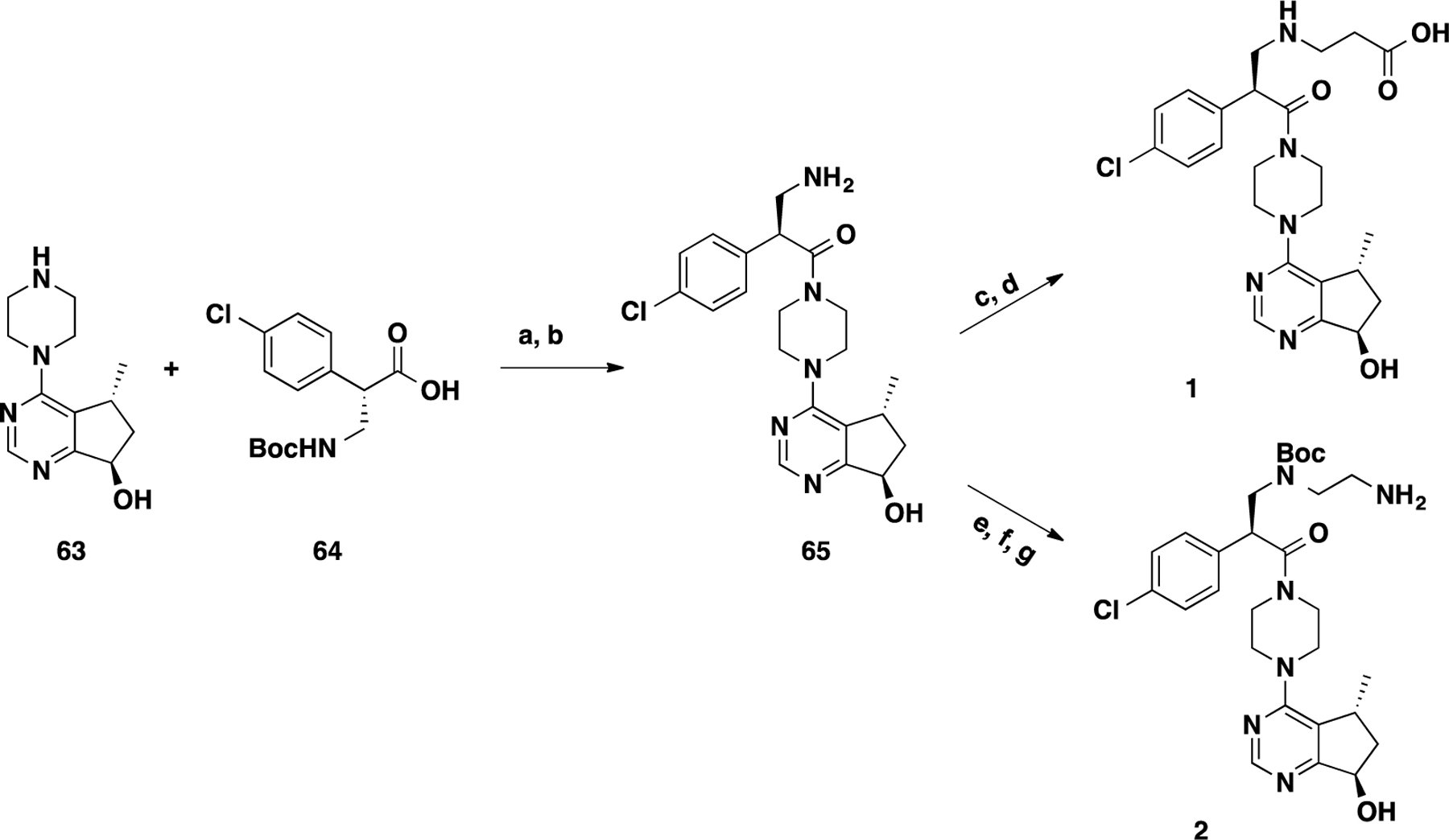
aReaction conditions: (a) 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI), 1-hydroxy-7-azabenzo-triazole (HOAt), N-methylmorpholine (NMM), DMSO, rt, 12 h; (b) TFA/DCM, rt, 30 min; (c) ethyl 3-bromopropanoate (66), K2CO3, DMF, 80 °C, 12 h; (d) LiOH, THF, H2O, rt, 12 h; (e) benzyl (2-iodoethyl)carbamate (67), K2CO3, CH3CN, 80 °C, 8 h; (f) Boc2O, Et3N, DCM, rt, 2 h; and (g) H2, Pd/C, MeOH, rt, 1 h.
