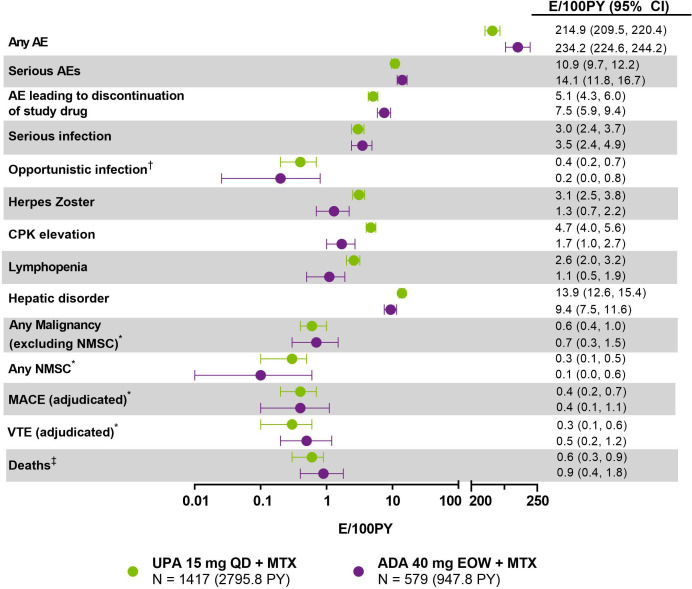
Figure 2.
Treatment-emergent adverse event summary through 156 weeks (E/100 PY (95% CI)). Data through 156 weeks include all patients receiving upadacitinib or adalimumab, including rescue groups, with assignment based on drug exposure at the time of event. The last week-156 visit was on 6 August 2020. Exposure-adjusted event rates (EAERs) are reported for all events. *Exposure-adjusted incidence rates (EAIRs) were the same as EAERs for malignancies, MACE and VTE. All events were considered AESIs except any AE, serious AEs, AE leading to discontinuation of study drug and deaths. †Opportunistic infections exclude herpes zoster, tuberculosis and oral candidiasis. Event rates for oral candidiasis and active tuberculosis were similar on upadacitinib and adalimumab (0.2 and 0.1 E/100 PY vs 0.3 and 0.2 E/100 PY, respectively). ‡Includes non-treatment emergent deaths. ADA, adalimumab; AE, adverse event; AESI, adverse event of special interest; CPK, creatine phosphokinase; EOW, every other week; MACE, major adverse cardiovascular event; MTX, methotrexate; NMSC, non-melanoma skin cancer; PY, patient-years; QD, once daily; UPA, upadacitinib; VTE, venous thromboembolism.