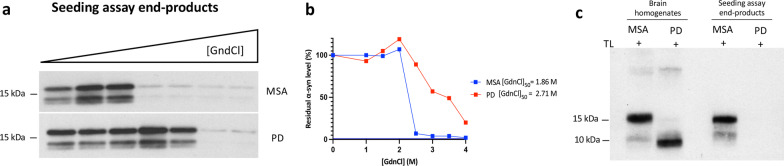
Fig. 5.
RT-QuIC reaction products seeded from MSA α-synuclein fibrils are conformationally distinguishable from RT-QuIC reaction products seeded from PD-derived fibrils. a, b Conformational stability assays for the RT-QuIC-derived MSA and PD fibrils. Representative α-synuclein immunoblots (a) and the resultant denaturation curves (b) are shown. MSA-derived fibrils are less stable than PD-derived fibrils in the epitope (amino acids 15–123) used to probe the structure. c Immunoblots of PBS-soluble α-synuclein species in brain homogenates from PD and MSA patients and their RT-QuIC-derived fibrils with thermolysin (TL) digestion. TL-resistant α-syn species were present in MSA and PD brain extracts, but TL-resistant α-syn species were only detectable in the MSA RT-QuIC-derived fibrils