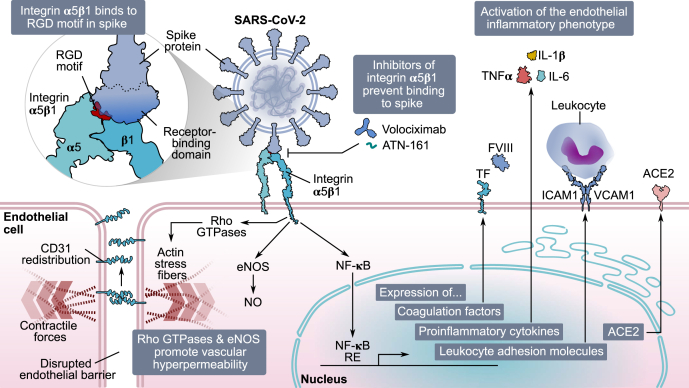
Figure 8.
Spike protein of SARS-CoV-2 causes endothelial dysfunction through integrinα5β1. In EC, integrin α5β1 recognizes the RGD motif in the receptor-binding domain of SARS-CoV-2 spike protein. Spike binding activates members of the RhoA family, CD31 redistribution, actin stress fibers formation, and eNOS phosphorylation, all of which contribute to a hyperpermeable endothelium. Moreover, integrin α5β1 binding to spike activates NF-κB, which translocates into the nucleus and induces the expression of proinflammatory mediators (coagulation factors [TF and FVIII], proinflammatory cytokines [TNF⍺, IL-1β, and IL-6], leukocyte adhesion molecules [VCAM1 and ICAM1]), and ACE2. Inhibitors of integrin α5β1 (volociximab and ATN-161) block its binding to spike, preventing the activation of the vascular inflammatory phenotype. ACE2, angiotensin-converting enzyme 2; CD31, cluster of differentiation 31; EC, endothelial cell; eNOS, endothelial nitric oxide synthase; FVIII, factor VIII; ICAM1, intercellular cell adhesion molecule 1; IL-1β, interleukin 1β; IL-6, interleukin 6; RGD, arginine–glycine–aspartic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TF, tissue factor; TNF⍺, tumor necrosis factor alpha; VCAM1, vascular cell adhesion molecule 1.