Abstract
Neuroendocrine, behavioural and autonomic responses to stressful stimuli are orchestrated by complex neural circuits. The caudal nucleus of the solitary tract (cNTS) in the dorsomedial hindbrain is uniquely positioned to integrate signals of both interoceptive and psychogenic stress. Within the cNTS, glucagon‐like peptide‐1 (GLP‐1) and prolactin‐releasing peptide (PrRP) neurons play crucial roles in organising neural responses to a broad range of stressors. In this review we discuss the anatomical and functional overlap between PrRP and GLP‐1 neurons. We outline their co‐activation in response to stressful stimuli and their importance as mediators of behavioural and physiological stress responses. Finally, we review evidence that PrRP neurons are downstream of GLP‐1 neurons and outline unexplored areas of the research field. Based on the current state‐of‐knowledge, PrRP and GLP‐1 neurons may be compelling targets in the treatment of stress‐related disorders.
LINKED ARTICLES
This article is part of a themed issue on GLP1 receptor ligands (BJP 75th Anniversary). To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v179.4/issuetoc
Keywords: Gcg, glucagon‐like peptide‐1, HPA axis, NTS, preproglucagon, Prlh, prolactin‐releasing peptide, stress
Abbreviations
- ACTH
adrenocorticotropic hormone
- CCK
cholecystokinin
- CNS
central nervous system
- cNTS
caudal nucleus of the solitary tract
- CRH
corticotropin‐releasing hormone
- DbH
dopamine β hydroxylase
- GLP‐1
glucagon‐like peptide‐1
- GPR10
G protein‐coupled receptor 10
- HPA
hypothalamic–pituitary–adrenal
- LiCl
lithium chloride
- NPFF
neuropeptide FF
- PrRP
prolactin‐releasing peptide
- PVN
paraventricular nucleus of the hypothalamus
1. INTRODUCTION AND PURPOSE OF REVIEW
Faced with an ever‐changing environment, the brain continuously monitors and prioritizes the needs of the body and in response orchestrates adaptations in physiology and behaviour that often are essential for survival. Stressors, defined here as imminent actual or perceived threats to survival or well‐being, result in sympathetic arousal, suppression of feeding and other motivated behaviours, and activation of the hypothalamic–pituitary–adrenal (HPA) axis (Holt & Trapp, 2016; Ulrich‐Lai & Herman, 2009). Molecularly diverse populations of neurons within the spinal cord, brainstem, hypothalamus and limbic forebrain form complex neural circuits that orchestrate stress responses (Ulrich‐Lai & Herman, 2009) and our understanding of the components of these circuits and how they are organized is constantly expanding. In this article we review the current state of knowledge about two specific populations of stress‐sensitive neurons located in the caudal nucleus of the solitary tract (cNTS), positioned within the dorsomedial hindbrain.
The first section of our review introduces the cNTS as an essential node in the brain's response to both interoceptive and psychogenic stress. This is followed by a brief introduction to two specific and discrete populations of neurons contained within the cNTS (i.e. glucagon‐like peptide‐1 (GLP‐1)‐ and prolactin‐releasing peptide (PrRP)‐expressing neurons) and a summary of emerging anatomical and functional evidence supporting their role in centrally‐mediated stress responses. We next discuss the possibility that GLP‐1 and PrRP neurons interact to modulate behavioural and physiological stress responses. Finally, we consider the future directions of this research field and the translational implications of the evidence discussed. Although GLP‐1 and PrRP also are expressed in some peripheral tissues, the current review is focused on neural cell populations found in the cNTS. Thus, we do not discuss peripheral (i.e. intestinally‐derived) GLP‐1 signalling, which is quite distinct from central GLP‐1 neural signalling (see this issue of British Journal of Pharmacology) (Brierley et al., 2021; Holt et al., 2020). We also do not in detail review the role of hindbrain GLP‐1 and PrRP neurons in the regulation of food intake and body energy balance. Those important topics are discussed in depth in other articles within this themed issue of British Journal of Pharmacology and elsewhere (Dodd & Luckman, 2013; Gribble & Reimann, 2021; Grill, 2020).
2. THE CAUDAL NUCLEUS OF THE SOLITARY TRACT—AN ESSENTIAL NODE FOR RESPONSES TO INTEROCEPTIVE AND PSYCHOGENIC STRESSORS
The cNTS is considered the ‘visceral’ NTS, distinct from the more rostral ‘gustatory’ NTS and is a component of the dorsal vagal complex, which also includes the area postrema and the dorsal motor nucleus of the vagus. The dorsal vagal complex is a critical central node for relaying interoceptive visceral, hormonal and somatic feedback from body to brain (Andresen & Mendelowitz, 1996; Grill & Hayes, 2009; Rinaman, 2007, 2010; Zhang et al., 2010). Predominant sources of relayed and direct interoceptive sensory inputs to the cNTS arise from spinal afferents, from vagal and glossopharyngeal afferents serving most visceral tissues, from hypothalamic neurons and from neurons within the area postrema that lie outside the blood–brain barrier and can detect toxins and other circulating factors that signal threats to homeostasis (Andresen & Mendelowitz, 1996; Maniscalco & Rinaman, 2017; Rinaman, 2010). These diverse sources of input position the cNTS to integrate multimodal signals regarding metabolic state and to monitor other changes in the internal environment, including changes that signal a threat to physiological homeostasis. Thus, the cNTS is ideally positioned to monitor the moment‐to‐moment status of the body's physiological systems. Further, axonal projections from the cNTS to every subcortical division of the CNS provide routes through which cNTS neurons can initiate and modulate behavioural, endocrine and autonomic responses to stressors that challenge or threaten to challenge these physiological systems.
2.1. Interoceptive stressors
Interoceptive stressors generate signals that mark and the actual current threat to physiological well‐being. For example, increased levels of circulating pro‐inflammatory cytokines can signal an infection, unloading of cardiac baroreceptors can signal haemorrhage, and increased levels of free fatty acids coupled with falling insulin can signal starvation. Interoceptive stressors and the signals they generate are strikingly similar across mice, rats, humans and other mammalian species, and behavioural and physiological stress responses to these signals do not require experience or learning. Signals of interoceptive threat originate within the body and are conveyed to the CNS largely through sensory pathways that converge onto cNTS neurons (see above), which in turn modulate the activity of (1), reticular and spinal central pattern generators and pre‐motor circuits that control behavioural outflow (Grill & Hayes, 2009), (2) parasympathetic neurons in the dorsal motor vagal nucleus and nucleus ambiguus (Broussard et al., 1998; Rinaman et al., 1989; Travagli et al., 2006) and (3) sympathetic neurons via inputs to the hindbrain reticular formation and spinal cord (Llewellyn‐Smith et al., 2015; Mtui et al., 1993; Zoccal et al., 2014). In addition, some cNTS neurons, which are sensitive to interoceptive stress signals, project to hypothalamic endocrine neurons to stimulate pituitary release of oxytocin, adrenocorticotropic hormone (ACTH) and other hormones, whose circulating levels increase in rodents after stress exposure (reviewed in Maniscalco & Rinaman, 2017; Rinaman, 2007, 2010; Ulrich‐Lai & Herman, 2009).
2.2. Psychogenic stressors
Psychogenic stressors generate signals that portend a potential threat to physiological well‐being; such threats can vary widely across mammalian species. Some psychogenic stressors and responses to them are innate (i.e. genetically coded), while others require experience‐based learning. For example, while laboratory rats and mice typically have never experienced a natural predator, they respond innately to predator signals (e.g. odours and visual cues) by mounting stress responses that prepare to meet an impending threat to survival. Other sensory stimuli can become psychogenic stressors through conditioning, for example mice and rats display endocrine, autonomic and behavioural stress responses to previously neutral auditory, olfactory or visual stimuli after they are paired with footshock, resulting in conditioned fear. Learned stress responses require neural processing by thalamic and cortical regions that integrate multiple sensory modalities in coordination with hippocampal and prefrontal cortical regions that serve mnemonic and executive functions. Importantly, and similar to interoceptive stressors, psychogenic stressors also activate neurons within the cNTS (Furlong et al., 2014; Holt, Pomeranz, et al., 2019; Maniscalco et al., 2015; Maniscalco & Rinaman, 2017). However, while most interoceptive stressors recruit cNTS neurons via interoceptive ‘bottom‐up’ signalling pathways, psychogenic stressors recruit cNTS neurons through descending projections from brain regions that include the medial prefrontal cortex, central nucleus of the amygdala, bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus (PVN) (Dayas et al., 2004; Dayas, Buller, Crane, et al., 2001; Dayas et al., 2001; Li et al., 1996; McKlveen et al., 2015; Ulrich‐Lai & Herman, 2009; van der Kooy et al., 1984). Stressor‐induced recruitment of these descending inputs to the cNTS mediate the ability of psychogenic stressors to suppress eating, to reduce vagal tone and to increase sympathetic outflow (see Sections 4.3, 4.5). Interestingly, neuroendocrine and emotional/affective responses to at least some psychogenic stressors require ascending projections from the cNTS back to the hypothalamus and limbic forebrain (Maniscalco & Rinaman, 2017). Thus, the cNTS is a critical network hub that receives not only interoceptive signals of threat to homeostasis, but also top‐down psychogenic stress signals; in both cases, efferent projections from the cNTS appear integral for generating and modulating behavioural, autonomic and endocrine stress responses.
2.3. Cell types within the caudal nucleus of the solitary tract—Focus on glucagon‐like peptide‐1 and prolactin‐releasing peptide neurons
The cNTS houses a number of intermixed populations of neurons defined by distinct connectional and molecular phenotypes. As reviewed elsewhere (Andresen et al., 2007; Maley, 1996; Riche et al., 1990; Rinaman, 2007, 2010; Sawchenko et al., 1990), different subpopulations of cNTS neurons express different combinations of amino acid, peptide and biogenic amine signalling molecules. These molecularly distinct cNTS subpopulations include locally‐projecting GABAergic neurons involved in hindbrain vagal sensory‐motor functions and projection neurons that target spinal, brainstem, hypothalamic and limbic forebrain regions implicated in behavioural and physiological stress responses. Of these various neural populations, two stress‐sensitive populations of particular interest (and the subject of this review) project both locally and to distant stress‐related CNS targets: GLP‐1 and PrRP neurons. GLP‐1‐ and PrRP‐positive neurons are restricted to the cNTS and one or two additional discrete locations, as described in the following section.
3. GLP‐1 AND PRRP NEURON DISTRIBUTION: OVERLAPPING BUT DISTINCT POPULATIONS IN THE CNTS
The distribution of GLP‐1 and PrRP neurons in the rodent brain is shown in Figure 1. GLP‐1‐immunopositive neurons reside within the medial and commissural subnuclei of the cNTS and within the intermediate reticular nucleus, positioned subjacent to the cNTS (Figure 1) (Llewellyn‐Smith et al., 2011; Maniscalco et al., 2015; Merchenthaler et al., 1999; Rinaman, 1999b). The cNTS and reticular GLP‐1 populations extend from the upper cervical spinal cord through the level of the mid‐area postrema (Llewellyn‐Smith et al., 2011). In rodents, an additional distinct group of GLP‐1 neurons is present in the olfactory bulb (Figure 1) (Merchenthaler et al., 1999; Thiebaud et al., 2019). Since these are locally‐projecting interneurons, GLP‐1 fibres and terminals throughout the rest of the CNS are assumed to originate from GLP‐1 neurons in the cNTS and reticular formation (Holt, Richards, et al., 2019; Llewellyn‐Smith et al., 2011; Thiebaud et al., 2019).
FIGURE 1.
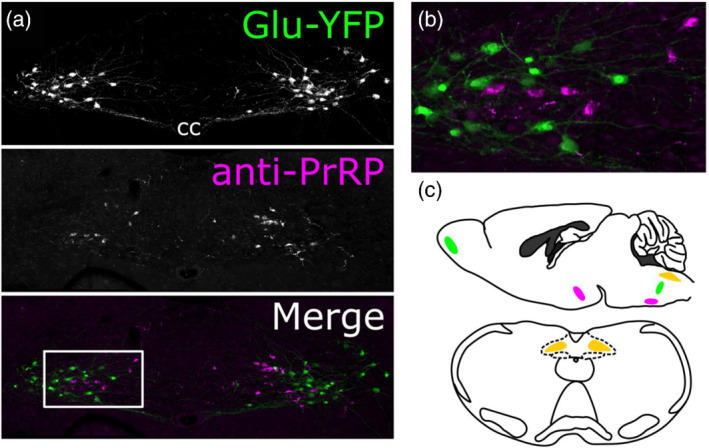
Expression of glucagon‐like peptide‐1 (GLP‐1) and prolactin‐releasing peptide (PrRP) in the mouse caudal nucleus of the solitary tract (cNTS). (a) Images of tissue from transgenic Glu‐YFP mice, expressing yellow fluorescent protein (YFP) under the control of the glucagon promoter, resulting in YFP in GLP‐1 neurons in the NTS (top panel, Hisadome et al., 2010). Tissue was immunolabelled for GFP (abcam ab13970, 1:5000) and PrRP (Phoenix H‐008‐52, 1:1000) to visualise cell bodies in the cNTS. (b) Inset from (a). (c) Schematic showing the localisation of the cNTS and PrRP and GLP‐1 neurons within it (orange) in a saggital (top) and horizontal (bottom) section from mouse brain. Additional regions with PrRP cell bodies (dorsomedial hypothalamus and ventrolateral medulla), which may contribute to terminal fields throughout the brain are indicated in magenta. GLP‐1 neurons are also found in the medullary intermediate reticular nucleus and olfactory bulb (indicated in green). However, olfactory bulb GLP‐1 neurons are interneurons and as such are presumed not to contribute to GLP‐1 terminal fields in the rest of the brain
PrRP, a neuropeptide originally named for its ex vivo stimulatory effect on prolactin release from the isolated anterior pituitary (Hinuma et al., 1998; Lawrence et al., 2000), is expressed not only by medial and commissural cNTS neurons, but also by neurons within the dorsomedial hypothalamus and the ventrolateral medulla (Figure 1) (Hinuma et al., 1998; Iijima et al., 1999; Maruyama et al., 1999; Morales et al., 2000; Roland et al., 1999). PrRP neurons within the cNTS comprise a subpopulation of the tyrosine hydroxylase (TH)‐ and dopamine β hydroxylase (DBH)‐positive A2 noradrenergic cell group (Morales et al., 2000), such that the majority of A2 neurons positioned caudal to the rostrocaudal level of the mid‐area postrema co‐express PrRP (Ellacott et al., 2002; Maruyama et al., 2001).
Importantly, although GLP‐1‐ and PrRP‐expressing neurons are similarly distributed within the cNTS, they comprise two distinct populations (Figure 1). PrRP neurons do not express GLP‐1 (Card et al., 2018; Morales et al., 2000) and GLP‐1 neurons do not express either PrRP (Card et al., 2018) or TH (Hisadome et al., 2010; Maniscalco et al., 2012). Published evidence in rats indicates that most caudal A2 neurons (the majority of which are PrRP‐positive) and most or all GLP‐1 neurons are glutamatergic (Stornetta et al., 2002; Zheng et al., 2015). The glutamatergic phenotype of GLP‐1 neurons also has been confirmed in mice (Liu et al., 2017).
3.1. Central targets of glucagon‐like peptide‐1 (GLP‐1) and prolactin‐releasing peptide (PrRP) neurons
In general, the central distribution of GLP‐1‐immunopositive fibres and terminals (which arise exclusively from neurons within the cNTS and closely adjacent medullary reticular formation) is largely similar to the central distribution of PrRP‐positive fibres and terminals. PrRP signals primarily through the G protein‐coupled receptor 10 (GPR10), although high affinity also has been demonstrated for the neuropeptide FF (NPFF) 2 receptor (Ma et al., 2009). GPR10 and GLP‐1 receptors are widely distributed throughout the brain, with high expression levels in stress‐relevant areas that contain GLP‐1‐ and PrRP‐positive fibres and terminals. These regions include but are not limited to the locus coeruleus, PVN and bed nucleus of the stria terminalis (Cork et al., 2015; Merchenthaler et al., 1999; Roland et al., 1999). It should be noted that while PrRP‐positive fibres arise from the cNTS A2 cell group, some fibres may also originate from the other two PrRP‐containing nuclei:‐ the ventral medullary A1 cell group and non‐catecholaminergic neurons in the dorsomedial nucleus of the hypothalamus (Figure 1c) (Morales et al., 2000). However, the axonal projections of hypothalamic PrRP neurons have not been reported.
The extensive axonal projections of hindbrain GLP‐1 neurons have been thoroughly mapped in both mice (Llewellyn‐Smith et al., 2011, 2013, 2015) and rats (Gu et al., 2013). These projection targets include many CNS regions implicated in physiological and behavioural responses to stress. As an example, within the brainstem, GLP‐1 fibres innervate the rostral ventrolateral medulla, the locus coeruleus, the parabrachial nucleus, the raphé pallidus and the periaqueductal grey (Llewellyn‐Smith et al., 2011, 2013). GLP‐1 neurons also innervate sympathetic preganglionic neurons in the spinal cord (Llewellyn‐Smith et al., 2015). Comparatively less is known regarding the brain‐wide distribution of PrRP‐positive fibres in rats and mice; initial studies reported PrRP immunolabeling only in rats (Iijima et al., 1999; Maruyama et al., 1999), without delineating the relative contributions of cNTS, ventrolateral medulla and dorsomedial hypothalamic PrRP neurons to terminal fields within various brain regions. One report documented an absence of PrRP immunolabeling within the lumbar dorsal horn and concluded that the rat spinal cord lacks PrRP fibres (Kalliomäki et al., 2004, however it is unclear whether all spinal levels were examined in that study.
Stress‐related forebrain regions that receive axonal input from the cNTS and also contain GLP‐1‐positive fibres, PrRP‐positive fibres and DbH‐positive (i.e. noradrenergic) fibres, which include the paraventricular thalamus, the PVN and the bed nucleus of the stria terminalis (Iijima et al., 1999; Llewellyn‐Smith et al., 2011; Maruyama et al., 1999). However, the relative density of GLP‐1‐ versus PrRP‐positive fibres can differ within these regions. For example, in rats, PrRP‐positive fibres are particularly dense within the anterior ventral bed nucleus of the stria terminalis and are more sparsely distributed within the anterior dorsal bed nucleus of the stria terminalis (Iijima et al., 1999; Maniscalco et al., 2015; Maruyama et al., 1999), whereas GLP‐1‐positive fibres are distributed more densely within the dorsal region of the anterior bed nucleus of the stria terminalis and are sparser within the ventral region (Gu et al., 2013; Rinaman, 2010). However, differences in innervation density are not necessarily linked to differences in the functional impact of innervation. In many cases, the postsynaptic targets of GLP‐1 and PrRP inputs to specific brain regions remain undefined and may include the distal dendrites and/or axons of target cells located outside of that region. In addition, neuropeptides are well‐established to signal through volume transmission, in which the peptide diffuses some distance and binds to receptors located far from the release site (van den Pol, 2012). Indeed, this phenomenon is suggested to underlie documented effects of GLP‐1 in the hippocampus (Hsu et al., 2015), a brain region that does not receive axonal input from GLP‐1 neurons.
In addition to multiple long‐range projection targets, GLP‐1 fibres are present within the cNTS, where some lie in close apposition to both PrRP and GLP‐1 neurons (Card et al., 2018). The existence of functional synaptic connections between these cell groups remains to be investigated. A moderate number of neurons within the cNTS express GLP‐1 receptors (Cork et al., 2015; Farkas et al., 2020; Jensen et al., 2018; Merchenthaler et al., 1999), including PrRP neurons in rats (Card et al., 2018), but excluding GLP‐1 neurons in mice (Card et al., 2018; Hisadome et al., 2010) and rats (Card et al., 2018). Thus, and as further discussed in Section 6 below, GLP‐1 signalling may participate in driving the activation of PrRP neurons within the cNTS.
4. A ROLE FOR CENTRAL PRRP AND GLP‐1 IN STRESS MODULATION?
We propose that three requirements must be met for a given neuropeptide to participate in modulating stress responses under physiological conditions:‐ (1) the activity of the cells producing the neuropeptide must be modulated in response to stress‐related stimuli, (2) the neuropeptide, when injected into the brain, should be able to elicit stress‐related behaviours and/or physiological responses and (3), endogenous neuropeptide signalling must contribute to the behavioural or physiological responses to stress. We argue that all three of these requirements are met by both GLP‐1 and PrRP neurons. In the following paragraphs we summarise evidence that PrRP and GLP‐1 neurons are broadly sensitive to physiologically and emotionally stressful stimuli (Table 1), that both PrRP and GLP‐1 administered centrally can elicit endocrine, autonomic and behavioural responses associated with stress and that endogenous PrRP and GLP‐1 signalling are necessary for a number of these stress responses.
TABLE 1.
Stimuli which activate GLP‐1, PrRP and A2 neurons in the cNTS determined from either cFos (or pSTAT3 for leptin) immunoreactivity, patch‐clamp electrophysiology (ex vivo), or calcium imaging (ex vivo)
| Stress‐related stimulus | GLP‐1 neurons | PrRP neurons | A2 neurons | References |
|---|---|---|---|---|
| CCK (high dose, 5–100 μg, i.p.) | + | + | + | (Hisadome et al., 2011; Holt et al., 2017; Lawrence et al., 2002; Maniscalco et al., 2020; Maniscalco & Rinaman, 2013; Rinaman, 1999b; Rinaman et al., 1995; Wall et al., 2020) |
| LiCl (0.15 M, 2% BW, i.p.) | + | + | + | (Lachey et al., 2005; Lawrence et al., 2002; Rinaman, 1999b) |
| LPS (100μg, i.p.) | + | ND | + | (Bienkowski & Rinaman, 2011; Gaykema et al., 2009; Rinaman, 1999b) |
| IL‐6 (ex vivo) | + | ND | ND | (Anesten et al., 2016) |
| Haemorrhage (18–25% blood loss) | ND | + | + | (Dun et al., 1993; Uchida et al., 2010) |
| Footshock | ND | + | + | (Morales & Sawchenko, 2003; Zhu & Onaka, 2002) |
| Restraint | + | + | + | (Adachi et al., 2005; Maniscalco et al., 2015; Maruyama et al., 2001; Palkovits et al., 1997; Sun et al., 2005; Terrill et al., 2019) |
| Elevated platform exposure | + | + | + | (Maniscalco et al., 2015) |
| Conditioned fear | − | + | + | (Edwards et al., 2021; Yoshida et al., 2014; Zhu & Onaka, 2002, 2003) |
| Non‐stress‐related stimulus | ||||
| Gastric distension | + | ND | + | (Vrang et al., 2003) |
| CCK (low dose, 1–3 μg) | − | + | + | (Maniscalco & Rinaman, 2013; Wall et al., 2020) |
| CCK (ex vivo) | + | ND | ND | (Hisadome et al., 2011; Holt et al., 2017) |
| Oxytocin | + (ex vivo) | ND | + | (Brierley et al., 2021; Ho et al., 2014) |
| Sweetened milk | + | ND | + | (Gaykema et al., 2009) |
| 5‐HT (ex vivo) | + | ND | ND | (Holt et al., 2017) |
| Leptin (in vivo and GLP) |
+ (mouse); −(rat) |
− (mouse) | +/− (rat) | (Dodd et al., 2014; Hisadome et al., 2010; Holt et al., 2017; Huo et al., 2008; Maniscalco & Rinaman, 2014; Williams et al., 2009) |
| Feeding | + | + | + | (Kreisler et al., 2014; Kreisler & Rinaman, 2016) |
| Leucine | ND | + | ND | (Tsang et al., 2020) |
Note: For leptin, significant species differences have been reported. for clarity, the species is indicated. In a few instances, there were discrepancies in the literature, which is indicated by +/−. TH‐positive A2 neurons are included, because they include PrRP neurons and these data provide important insight when data is lacking for PrRP neurons. ND: Not determined.
4.1. cFos labelling as a marker of stimulus‐induced ‘activation’
In published studies examining stress‐induced recruitment of central neural circuits, including our own, stimulus‐induced ‘activation’ of neurons within the cNTS and other central regions is often determined through immunohistochemical localization of nuclear cFos. cFos is the product of the immediate‐early response gene, cfos, which is activated transiently and rapidly in response to neural stimulation. The design in most of these studies includes exposing an animal to an experimental treatment or stimulus with a controlled onset, followed by tissue harvest 1–2 h later in order to visualise cFos immunolabeling in regions of interest. As a binary index of neural activation, the presence or absence of visible cFos labelling poorly reflects the degree of activation and provides no information about stimulus‐induced changes in spike frequency. Nonetheless, quantitative analyses of neural cFos expression in animals exposed to experimental versus control conditions permit assessment of stimulus‐induced activation of phenotypically identified neurons in defined anatomical regions, making it an ideal approach for testing hypotheses regarding neural sensitivity and function in vivo.
4.2. Prolactin‐releasing peptide (PrRP) and glucagon‐like peptide‐1 (GLP‐1) neurons are co‐activated following interoceptive and psychogenic stress
Extensive quantitative analyses of cFos expression have revealed that a variety of acute psychogenic stressors activate PrRP and GLP‐1 neurons in rats and mice (Holt & Trapp, 2016; Maniscalco & Rinaman, 2017; Morales & Sawchenko, 2003). Substantial evidence also indicates that interoceptive stressors activate GLP‐1 neurons in both rodent species, whereas conclusions regarding the sensitivity of PrRP neurons to interoceptive stress are less secure. For example, exogenously administered cholecystokinin (CCK) at doses exceeding 5 μg·kg−1 body weight (BW) serves as an interoceptive stressor that activates the HPA stress axis in a dose‐dependent manner in rats (Kamilaris et al., 1992) and such doses activate both GLP‐1 and PrRP neurons in rats and mice (Lawrence et al., 2002; Maniscalco et al., 2020; Maniscalco & Rinaman, 2013; Rinaman, 1999b). PrRP signalling via GPR10 contributes to the hypophagic effect of CCK administered at 15 μg·kg−1 in mice (Bechtold & Luckman, 2006), although concurrent CCK‐induced activation of the HPA axis in mice and the contribution of GPR10 to that potential activation has not been reported. The nauseogenic agent lithium chloride (LiCl; 0.15 M, 2% bodyweight) and the pro‐inflammatory cytokine stimulus lipopolysaccharide (LPS, 100 μg·kg−1) represent two additional interoceptive stressors that robustly activate GLP‐1 neurons in rats (Rinaman, 1999b). LPS‐induced activation of PrRP neurons has not been reported, but LPS treatment suppresses food intake similarly in wildtype and GPR10 knockout mice (Bechtold & Luckman, 2006), suggesting that PrRP signalling is unnecessary for the stress‐related hypophagic response to LPS. In addition, the same dose of LiCl that activates GLP‐1 neurons in rats and mice (Lachey et al., 2005; Rinaman, 1999b) was reported to not elicit cFos expression in PrRP neurons in rats (Lawrence et al., 2002). Based on those findings, Luckman and colleagues proposed that PrRP neurons are not activated by interoceptive stressors in general. However, PrRP neurons within the rat cNTS are activated by hypotensive haemorrhage, a powerful interoceptive stressor (Morales & Sawchenko, 2003). It will be important to confirm interoceptive stressor‐specific neural responses (or lack thereof) in both rats and mice, and to determine whether PrRP neurons in one or both rodent species are recruited in response to other types of interoceptive stress.
Several lines of evidence support the view that endogenous GLP‐1 signalling contributes to a broad range of neural responses to interoceptive stress. Central delivery of GLP‐1 receptor agonists in rats elicits conditioned flavour avoidance and pica, two behavioural responses typically observed following malaise‐inducing interoceptive stress (Kinzig et al., 2002; van Dijk & Thiele, 1999). In addition, central GLP‐1 signalling in rats is necessary for LPS‐ and LiCl‐induced hypophagia (Grill et al., 2004; Lachey et al., 2005; Rinaman, 1999a), and also is necessary for the ability of LiCl to support conditioned taste avoidance (Seeley et al., 2000), to suppress dopamine release in the nucleus accumbens (Fortin et al., 2016) and to activate the HPA axis (Kinzig et al., 2003). Conversely, central administration of exogenous PrRP in rats does not support conditioned flavour avoidance and suppresses food intake without disrupting the behavioural satiety sequence (Lawrence et al., 2002). The latter finding suggests that central PrRP‐induced hypophagia reflects engagement of physiological satiety mechanisms rather than simply disrupting ingestive behaviours. Thus, the collective results from studies using rats indicate that stress and malaise contribute to the hypophagic effect of centrally administered GLP‐1 (Kinzig et al., 2002; van Dijk & Thiele, 1999), but not to the hypophagic effect of centrally administered PrRP (Lawrence et al., 2002). On the other hand, knockdown of either PrRP or GLP‐1 expression within the cNTS rescues bodyweight loss and hypophagia in a rat tumour growth model, evidence that both neuropeptides contribute to cancer‐induced anorexia (Borner et al., 2018; Navarro I Batista et al., 2020). Thus, while the rat data clearly implicate central GLP‐1 signalling in hypophagia associated with stress and malaise, additional research is needed to resolve the basis of PrRP‐induced hypophagia.
A potentially important species difference in the role of central GLP‐1 signalling is worth noting here. As mentioned above, GLP‐1 neurons are activated in both rats (Lachey et al., 2005; Rinaman, 1999b) and mice (Lachey et al., 2005) following LiCl treatment. However, while blockade of central GLP‐1 signalling reduces the hypophagic effect of LiCl in rats (Rinaman, 1999a), GLP‐1 receptor signalling is not required for LiCl‐induced hypophagia or conditioned taste aversion in mice (Lachey et al., 2005). Furthermore, and similar to the pharmacological effects of exogenous PrRP delivered into the brain in rats, chemogenetic activation of GLP‐1 neurons in mice suppresses food intake without disrupting the behavioural satiety sequence and does not support conditioned flavour avoidance (Brierley et al., 2021; Gaykema et al., 2017). Results from parallel chemogenetic studies in rats have not been reported, but the chemogenetic data in mice are inconsistent with earlier pharmacological studies in rats and mice, in which central administration of GLP‐1 or agonist does support conditioned flavour avoidance (Kinzig et al., 2002; Lachey et al., 2005; Rinaman, 1999a). Additional research will be necessary to reconcile these differences, but the available data suggest that central GLP‐1 signalling plays a larger role in behavioural responses to malaise‐inducing interoceptive stressors in rats than in mice.
As indicated at the beginning of this section, GLP‐1 and PrRP neurons within the cNTS are highly responsive to psychogenic stressors. For example, restraint stress activates GLP‐1 neurons in both rats and mice (Holt, Richards, et al., 2019; Maniscalco et al., 2015; Terrill et al., 2019). Similarly, restraint activates PrRP neurons in rats (Adachi et al., 2005; Maruyama et al., 2001; Sun et al., 2005) and increases PrRP mRNA expression within the rat cNTS (Mera et al., 2007). PrRP neurons also express cFos in rats following acute footshock (Morales & Sawchenko, 2003), following brief exposure to an elevated open platform (Maniscalco et al., 2015) or following exposure to conditioned fear cues (Edwards et al., 2021; Yoshida et al., 2014; Zhu & Onaka, 2003). Conversely, while GLP‐1 neurons also are activated in rats after elevated platform exposure (Maniscalco et al., 2015), exposure of rats to conditioned fear cues (e.g. previously neutral cues that were paired with footshock) activates PrRP but not GLP‐1 neurons within the cNTS (Edwards et al., 2021; Maniscalco et al., 2015). As such, conditioned fear cues appear to be the only type of psychogenic stress stimulus examined thus far which does not lead to co‐activation of PrRP and GLP‐1 neurons in rats. Given the otherwise overwhelming overlap in GLP‐1 and PrRP neural sensitivity to psychogenic stressors, the differential responses of these neurons to conditioned fear cues is somewhat surprising. However, as compared to unconditioned primary stressors (e.g. restraint or footshock), conditioned fear cues and other types of secondary stressors are likely to engage different, if partially overlapping, central pathways. Further research is needed to understand the potential implications of the relatively higher sensitivity of PrRP versus GLP‐1 neurons to conditioned stressors.
With the exceptions noted above, the available evidence indicates that GLP‐1 and PrRP neurons within the cNTS generally are co‐activated after several distinct types of interoceptive and psychogenic stress, suggesting that these neural populations share similar inputs and/or that one neural population is functionally ‘downstream’ of the other. The idea that one cell population recruits the other is discussed in more detail in Section 6. Importantly, while neural activation (i.e. cFos expression) in response to stressful stimuli indicates that such stimuli recruit GLP‐1 and PrRP neurons, that evidence alone does not address the question of whether peptide signalling molecules released from these neurons are necessary to drive behavioural and physiological responses to stress. The literature summarized below suggests that the answer to this question often is yes.
4.3. Hypothalamic–pituitary–adrenal axis modulation by prolactin‐releasing peptide and glucagon‐like peptide‐1 (GLP‐1)
Activation of the endocrine HPA axis is a hallmark response to both psychogenic and interoceptive stressors (Ulrich‐Lai & Herman, 2009). Corticotropin‐releasing hormone (CRH)‐expressing neurons in the parvocellular PVN drive the release of ACTH from the anterior pituitary into the blood. ACTH elicits synthesis of corticosterone (cortisol in humans) within the adrenal cortex, which then enters the circulation. There is solid evidence to support a role for both PrRP and GLP‐1 in stimulating HPA axis activity. The axon terminals of PrRP and GLP‐1 neurons appear to synapse directly onto CRH neurons within the PVN (Bechtold & Luckman, 2006; Larsen et al., 1997; Liu et al., 2017; Maruyama et al., 2001; Matsumoto et al., 2000; Sarkar et al., 2003) and these CRH neurons express receptors for GLP‐1 (Li et al., 2019) and PrRP (Dodd & Luckman, 2013; Takayanagi & Onaka, 2010). This is evidence that both cNTS neural populations are well positioned to drive the HPA axis. In fact, A2 and GLP‐1 neurons together make up the majority of cNTS neurons that are retrogradely labelled from the PVN (Riche et al., 1990; Rinaman et al., 1995; Sawchenko & Swanson, 1982). PrRP activates CRH neurons ex vivo and in vivo. Firstly, PrRP decreases miniature GABAergic postsynaptic currents in parvocellular PVN neurons (Ma et al., 2009), likely including the CRH subpopulation. Secondly, CRH is released from hypothalamic explants in response to PrRP (Seal et al., 2002), and thirdly, intracerebroventricular (i.c.v.) injection of PrRP leads to cFos expression in CRH neurons (Bechtold & Luckman, 2006; Matsumoto et al., 2000). Similarly, GLP‐1 activates PVN CRH neurons ex vivo and in vivo: (1) GLP‐1 receptor signalling increases the firing frequency of PVN neurons (Acuna‐Goycolea & van den Pol, 2004), (2) optogenetic activation of GLP‐1 neuron terminals elicits excitatory postsynaptic currents in PVN CRH neurons (Liu et al., 2017) and (3) chemogenetic activation of GLP‐1 neurons, as well as i.c.v. injection of GLP‐1, leads to cFos expression in CRH neurons (Larsen et al., 1997; Liu et al., 2017). Additionally, i.c.v. administration of either neuropeptide leads to increased plasma levels of ACTH and corticosterone (Gil‐Lozano et al., 2010; Kinzig et al., 2003; Larsen et al., 1997; Matsumoto et al., 2000; Mera et al., 2007; Seal et al., 2002), effects which are mediated by CRH signalling (Gil‐Lozano et al., 2010; Matsumoto et al., 2000). Interestingly, HPA axis activation is enhanced by central co‐administration of noradrenaline and PrRP (Maruyama et al., 2001; Uchida et al., 2010), suggesting that these co‐expressed transmitters act in concert to increase stress hormone release. A similar interaction has been reported between GLP‐1 and the major co‐transmitter of GLP‐1 neurons, glutamate: patch clamp electrophysiological recordings and evidence from behavioural experiments suggest that GLP‐1 signalling enhances trafficking of AMPA/Kainate receptors into the membrane of CRH neurons in the PVN, thereby facilitating glutamatergic signalling (Liu et al., 2017).
Several additional lines of evidence suggest that endogenous GLP‐1 and PrRP signalling contributes to stress‐induced activation of the HPA axis. First, central injection of PrRP antibodies is sufficient to prevent stress‐ or nociception‐induced activation of PVN neurons (Mera et al., 2006; Zhu & Onaka, 2003), supporting our findings that selective lesioning of noradrenergic cNTS neurons with axonal inputs to the PVN (which include PrRP inputs) attenuates PVN activation and reduces plasma corticosterone levels in rats following CCK, LPS or LiCl treatment (Bienkowski & Rinaman, 2008; Rinaman, 2003; Rinaman & Dzmura, 2007). Second, mice lacking PrRP display no increase in plasma ACTH following re‐exposure to a conditioned fear stimulus (Yoshida et al., 2014) and GPR10 knockout mice have reduced levels of plasma corticosterone following a hypoglycaemic challenge (Laurent et al., 2005). Conversely, PrRP knockout mice displayed normal increases in PVN cFos and plasma ACTH, but blunted corticosterone release, in response to restraint stress (Mochiduki et al., 2010). These seemingly inconsistent findings indicate that additional research will be required to clarify the role of endogenous PrRP in HPA axis regulation. Regarding GLP‐1, mice lacking GLP‐1 receptors within the PVN display reduced plasma corticosterone responses following restraint stress (Ghosal et al., 2017), whereas whole‐organism knockout of Glp1r promotes increased plasma corticosterone responses to stress (Maclusky et al., 2000), suggesting that the effect of GLP‐1 signalling on corticosterone levels depends on the site of action. A relatively recent study found that chemogenetic activation of GLP‐1 neurons was by itself insufficient to increase plasma levels of corticosterone in mice (Gaykema et al., 2017). Interestingly, selective knockdown of Glp1r in the bed nucleus of the stria terminalis enhanced the corticosterone response to restraint stress in rats, suggesting that GLP‐1 signalling in this nucleus may provide a negative feedback signal that limits the extent or duration of the neuroendocrine stress response (Zheng et al., 2019). Considered together, these findings provide compelling yet inconclusive evidence that central GLP‐1 and PrRP signalling pathways contribute to stress‐induced HPA axis activation.
4.4. Sympathetic activation by glucagon‐like peptide‐1 (GLP‐1) and prolactin‐releasing peptide (PrPP)
Following exposure to a current or imminent threat, the brain rapidly increases sympathetic outflow and suppresses vagal tone, resulting in increased heart rate and blood pressure, hyperthermia and hyperglycaemia. Similarly, administration of either GLP‐1 or PrRP into the brain increases heart rate, blood pressure and body temperature (Barragán et al., 1999; Beiroa et al., 2014; Davis & Grill, 2018; Ellacott et al., 2003; Horiuchi et al., 2002; Lawrence et al., 2000, 2004; Lee et al., 2018; Lockie et al., 2012; Samson et al., 2000; Yamada et al., 2009; Yamamoto et al., 2002), most likely via a combination of reduced vagal tone and increased sympathetic outflow (Baggio et al., 2017; Ellacott et al., 2003; Griffioen et al., 2011; Holt et al., 2020; Lee et al., 2018; Yamamoto et al., 2002). Similarly, chemogenetic activation of GLP‐1 neurons increases heart rate in anaesthetised and in awake, behaving mice (Holt et al., 2020), and direct application of GLP‐1 to the thoracic spinal cord (T7‐T8) is sufficient to increase heart rate in mice (Holt et al., 2020). Thus, the known spinal projections of cNTS GLP‐1 neurons may serve to increase sympathetic outflow (Llewellyn‐Smith et al., 2015), although it is likely not the only contributing pathway. The cardiovascular effects of PrRP appear to be dependent on downstream signalling through CRH neurons, as well as PrRP signalling via the neuropeptide FF2 receptor rather than through GPR10 (Ma et al., 2009; Yamada et al., 2009), although interpretation of these pharmacological data should be strengthened by using selective GPR10 and neuropeptide FF2 receptor knockout strategies. Microinjection of PrRP into the ventrolateral medulla is sufficient to increase heart rate, blood pressure and sympathetic nerve activity in rats (Horiuchi et al., 2002). Interestingly, chronic ablation or acute chemogenetic inhibition of GLP‐1 neurons has no measurable effect on baseline heart rate or mean arterial blood pressure in mice (Holt et al., 2020), whereas PVN GLP‐1 receptors mediate restraint stress‐induced increases in heart rate in rats (Ghosal et al., 2017). Thus, central GLP‐1 and PrRP signalling pathways serve to increase cardiovascular outflow and may do so specifically in response to stressful stimuli.
4.5. Role of central glucagon‐like peptide‐1 (GLP‐1) and prolactin‐releasing peptide (PrRP) in behavioural stress responses
Along with neuroendocrine and autonomic responses, stress elicits substantial changes in motivated behaviour, including suppressed feeding, reduced exploration and increased vigilance (Holt & Trapp, 2016; Maniscalco & Rinaman, 2017, 2018). When injected into the brain, both GLP‐1 and PrRP robustly suppress food intake (Lawrence et al., 2000; Turton et al., 1996). While the physiological significance of those pharmacological data are unclear, additional evidence points to a role for endogenous central GLP‐1 in stress‐induced hypophagia. For example, while disruption of GLP‐1 signalling from cNTS neurons does not alter ad libitum feeding at dark onset in mice (Brierley et al., 2021; Cheng et al., 2020; Holt, Richards, et al., 2019), central GLP‐1 receptor signalling is necessary for stress‐induced hypophagia in both rats and mice (Maniscalco et al., 2015; Terrill et al., 2018, 2019; Williams et al., 2018), and chemogenetic inhibition of GLP‐1 neurons rescues stress‐induced suppression in food intake in mice (Holt, Richards, et al., 2019). Given the availability of a transgenic Prlh‐Cre mouse (Dodd et al., 2014), future studies could use a similar chemogenetic approach to examine whether PrRP signalling from the cNTS also contributes to stress‐induced hypophagia. In this regard, chemogenetic inhibition of PrRP neurons could help confirm and extend published evidence that PrRP knockdown in the cNTS reduces cancer‐related hypophagia (Navarro I Batista et al., 2020), whereas LPS‐induced hypophagia persists in whole‐body GPR10 knockout mice (Bechtold & Luckman, 2006).
In addition to the apparent role of central GLP‐1 in stress‐induced hypophagia, GLP‐1 signalling is implicated in other stress‐relevant behaviours. For example, stimulation of GLP‐1 receptors in the central amygdala or supramammillary nucleus reduces the time that rats spend in the centre of an open field, interpreted as an increase in anxiety‐like avoidance behaviour (Kinzig et al., 2003; López‐Ferreras et al., 2020). Interestingly, Glp1r knockdown in the supramammillary nucleus produced the opposite (i.e. anxiolytic) effect in the open field assay in female rats, but not in males (López‐Ferreras et al., 2020). In another study, knockdown of Glp1r in the bed nucleus of the stria terminalis suppressed light‐enhanced acoustic startle responses in male rats (females were not examined) (Zheng et al., 2019), consistent with a reduced level of ‘anxiety’. Similar investigations have not been reported for PrRP, although one paper reported that whole‐body GPR10 knockout had no impact on the behaviour of mice in several tests designed to assess anxiety‐like behaviours (Laurent et al., 2005). Collectively these data point to a role for GLP‐1 in driving stress‐induced behavioural changes, while additional work will be necessary to explore a similar role for PrRP neurons.
5. AFFERENT INPUTS UNDERLYING PSYCHOGENIC STRESS‐INDUCED ACTIVATION OF GLP‐1 AND PRRP NEURONS
The neural pathways mediating the activation of PrRP and GLP‐1 neurons in response to psychogenic stress are largely unknown. Central sources of axonal input to the region of the cNTS that contains PrRP and GLP‐1 neurons are similar in rats and mice, and arise from every major division of the CNS (Holt, Pomeranz, et al., 2019; van der Kooy et al., 1984; Veening et al., 1984). We recently utilized Cre‐conditional viral tracing approaches in mice to identify sources of input that specifically target GLP‐1 neurons in the cNTS (Holt, Pomeranz, et al., 2019). Documented sources of direct synaptic input to GLP‐1 neurons include primary vagal afferents in the nodose ganglion, of which a large proportion express receptors for oxytocin (Brierley et al., 2021), other NTS neurons, spinal sensory neurons in the dorsal horn and neurons within the PVN, lateral hypothalamic area, parasubthalamic nucleus, lateral division of the central nucleus of the amygdala, bed nucleus of the stria terminalis, Barrington's nucleus, raphé nuclei, periaqueductal grey, Kölliker‐Fuse nucleus, area postrema and medullary reticular formation. Additional sources of indirect input to GLP‐1 neurons include the prefrontal cortex, hippocampal formation, thalamus, basomedial amygdala and spinal trigeminal nucleus. These polysynaptic inputs presumably are relayed through one or more of the aforementioned sources of monosynaptic input to GLP‐1 neurons. While the excitatory versus inhibitory nature of most of these inputs remains to be determined, neurons within the lateral hypothalamus, PVN, and Barrington's nucleus that target the cNTS were found to express cFos in mice after restraint stress (Holt, Pomeranz, et al., 2019), perhaps contributing to stress‐induced activation of postsynaptic GLP‐1 neurons. In this regard, neurons in the lateral central nucleus of the amygdala that provide synaptic input to the cNTS do not express cFos in mice after restraint stress. These neurons presumably are GABAergic, based on evidence in rats (Saha et al., 2000), and so their lack of activation is consistent with the concurrent state of GLP‐1 neural activation. Interestingly, however, neurons within the medial amygdala (which do not directly innervate the cNTS) are necessary for the ability of conditioned fear cues to activate PrRP neurons (Yoshida et al., 2014). This circuit from medial amygdala to PrRP neurons within the cNTS is not yet defined, but could include a relay through GLP‐1 neurons that receive polysynaptic input from the basomedial amygdala (Holt, Pomeranz, et al., 2019).
It will be important to identify central sources of input to the cNTS that commonly or uniquely target GLP‐1 and PrRP neurons. Studies using conditional viral tracing approaches to isolate sources of synaptic input to GLP‐1 neurons have not been performed in rats and similar studies examining inputs to PrRP neurons have not been performed in either species. We do know that vagal afferent neurons in the nodose ganglia provide direct synaptic input to GLP‐1 neurons (Brierley et al., 2021; Hisadome et al., 2010; Holt, Pomeranz, et al., 2019) and also to A2 neurons, the latter presumably including PrRP neurons (Appleyard et al., 2007; Rinaman et al., 1989), although it is not known whether individual vagal afferent neurons target both cNTS populations. In mouse in vitro slice preparation, glutamatergic visceral afferent signals produce tightly synced, large‐amplitude excitatory postsynaptic currents in A2 and GLP‐1 neurons, providing high‐fidelity transmission of sensory nerve activity (Appleyard et al., 2007; Hisadome et al., 2010).
6. ANATOMICAL AND FUNCTIONAL EVIDENCE FOR A DIRECT LINK BETWEEN GLP‐1 AND PRRP NEURONS
The evidence discussed above outlines a variety of central and peripheral sources of input to GLP‐1 and PrRP neurons that may mediate interoceptive and psychogenic stress‐induced activation of these cell populations. In addition, substantial local connectivity exists within the cNTS and the clear overlap in the sensitivity of GLP‐1 and PrRP neurons to stressful stimuli suggests that one neural population could be functionally downstream of the other. While this question has not been directly addressed using electrophysiological approaches, we summarise here previous findings pointing to a direct link between GLP‐1 and PrRP neurons.
In our recent study using rabies and pseudorabies viruses to map mono‐ and polysynaptic inputs to GLP‐1 neurons in mice (Holt, Pomeranz, et al., 2019), we found no evidence for either direct or indirect input arising from TH+ cNTS neurons, which would include PrRP neurons (Holt, Pomeranz, et al., 2019). It currently is unknown whether GLP‐1 neurons express the PrRP receptor (GPR10). Conversely, PrRP neurons express GLP‐1 receptor mRNA in rats (Card et al., 2018), consistent with other evidence that TH+ neurons within the cNTS express GLP‐1 receptors (Cork et al., 2015), and also consistent with evidence that TH+ neurons within the cNTS and PrRP‐positive neurons are closely apposed by GLP‐1 fibres and terminals (Card et al., 2018; Llewellyn‐Smith et al., 2013).
A limitation of both in situ hybridisation and genetic models is the inability to determine the subcellular localisation of the GLP‐1 receptor protein. Based on results using a now well‐validated GLP‐1 receptor antibody, 7F38A2 (Jensen et al., 2018), a recent report stated (albeit without showing the evidence) that GLP‐1 receptor protein was not colocalized with PrRP immunolabeling within the rat cNTS (Gabery et al., 2020). It is possible that GLP‐1 receptors do not accumulate in the soma but instead are transported to the distal processes of PrRP neurons. In support of this, a recent study of the ultrastructural localisation of GLP‐1 receptor protein demonstrated very little on cell bodies within the NTS of male rats (Farkas et al., 2020). While the collective data reviewed above indicate that PrRP neurons express GLP‐1 receptor mRNA and are anatomically poised to respond to GLP‐1 released by fibres and terminals in close proximity, it remains unclear whether such signalling occurs within the cNTS (e.g. at the cell body or local dendrites) and/or at distant locations where GLP‐1 and PrRP axon terminal fields are co‐distributed (e.g. within the PVN). An additional interesting question is whether the small population of PrRP‐expressing neurons in the dorsomedial hypothalamus express Glp1r and receive close appositions from GLP‐1 neurons. This is a compelling idea, based on three key pieces of evidence:‐ (1) the dorsomedial hypothalamus is perhaps the densest innervation target of NTS GLP‐1 neurons (Llewellyn‐Smith et al., 2011), (2) dorsomedial hypothalamic PrRP neurons drive thermogenesis (Dodd et al., 2014) and (3) dorsomedial hypothalamic GLP‐1 receptor stimulation increases body temperature (Lee et al., 2018). Future studies using transgenic Prlh‐Cre mice (Dodd et al., 2014) could help to address whether dorsomedial hypothalamic PrRP neurons mediate the thermogenic effects of central GLP‐1, perhaps playing a role in the thermogenic effects of acute stress.
In support of a functional role for a GLP‐1‐to‐PrRP signalling pathway, we reported that central (i.c.v.) administration of a selective GLP‐1 receptor antagonist (exendin‐9) significantly reduced the ability of restraint stress to activate PrRP neurons in rats (Maniscalco et al., 2015). The current lack of selective antagonists for GPR10 makes it challenging to similarly examine whether endogenous PrRP signalling can recruit GLP‐1 neurons and their downstream targets. However, indirect evidence addressing this issue is provided by the observation that neurochemically selective destruction of A2 neurons in the cNTS (including those that express PrRP) does not block the ability of a large systemic dose of CCK to activate GLP‐1 neurons (Rinaman, 2003). Considered together, these data support the view that while GLP‐1 and PrRP neurons appear to have many common sources of input, PrRP neurons also are downstream of GLP‐1 neurons, whereas the converse may not be true.
7. CONCLUSIONS AND FUTURE DIRECTIONS
We have presented here the current evidence that GLP‐1 and PrRP neurons are similarly sensitive to a wide range of interoceptive and psychogenic stressors, and that these neurons contribute to stress‐induced neuroendocrine, behavioural and autonomic changes (Figure 2). We discuss the hypothesis that PrRP neurons are likely to be downstream of GLP‐1 neurons, perhaps explaining their partially overlapping sensitivity to stressful stimuli and their generally similar functional contributions to stress responses. Based on decades of research, however, it is unlikely that GLP‐1 and PrRP neurons strictly and only are important under conditions of stress. Indeed, both cell populations respond to non‐stressful feeding‐related sensory feedback (Table 1) and both populations contribute to suppression of food intake under certain conditions that presumably are not stressful (Dodd & Luckman, 2013; Holt, Richards, et al., 2019). We also do not argue that PrRP and GLP‐1 neurons are necessary for coordinating all aspects of stress responsiveness. Instead, the evidence we presented help to establish working hypotheses regarding potentially unique roles played by these molecularly distinct populations of cNTS neurons, and how these roles are integrated within the broader and more complex array of neural systems that organize behavioural and physiological responses to real and perceived threats to homeostatic well‐being. While the role of endogenous GLP‐1 has been relatively well‐studied through genetic mouse models and the use of pharmacological, chemogenetic and optogenetic approaches, comparatively less is known regarding the physiological conditions during which endogenous PrRP signalling is important. The availability of Prlh‐Cre mice (Dodd et al., 2014) as well as a recently reported adeno‐associated virus for knocking down PrRP expression in rats (Navarro I Batista et al., 2020) will undoubtedly facilitate these investigations and may further elucidate the role of the apparent GLP‐1‐to‐PrRP pathway. In addition, the potential differential contribution of distinct subpopulations of cNTS PrRP and GLP‐1 neurons in different aspects of stress responses, that is, endocrine versus behavioural, is currently unknown.
FIGURE 2.
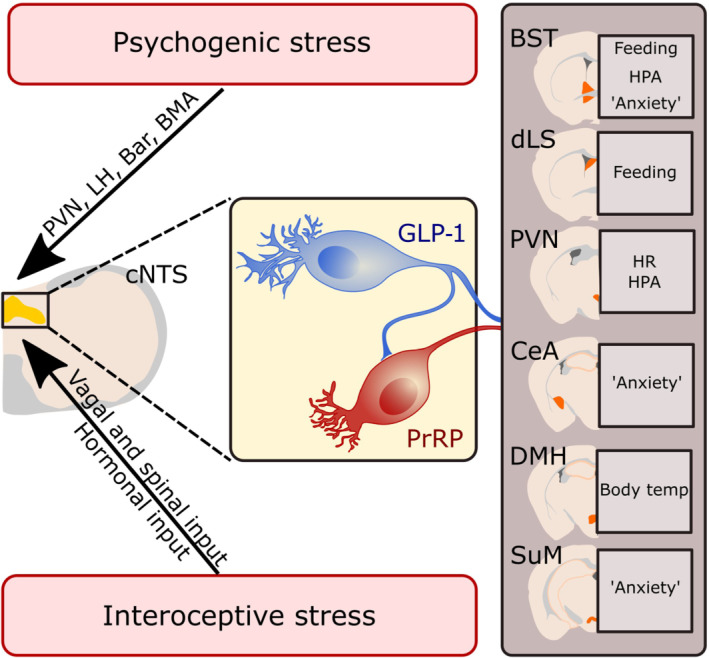
Proposed model for the role of prolactin‐releasing peptide (PrRP) and glucagon‐like peptide‐1 (GLP‐1) neurons in interoceptive and psychogenic stress responses. Interoceptive and psychogenic stressors activate neurons in the caudal nucleus of the solitary tract (cNTS), including both GLP‐1 and PrRP neurons. GLP‐1 neurons then further drive activation of PrRP neurons and PrRP and GLP‐1 signalling in distributed forebrain nuclei leads to suppression in feeding, HPA axis activation, increased heart rate (HR) and body temperature, and enhanced anxiety‐like behaviours. Displayed are regions in which endogenous GLP‐1 signalling has been demonstrated to contribute to the indicated stress‐related responses (see text for references). The lack of a selective antagonist for the receptor for PrRP (GPR10) has precluded parallel pharmacological studies focused on the role of endogenous PrRP signalling. Bar: Barrington's nucleus; BMA: Basomedial amygdala; BST: Bed nucleus of the stria terminals; CeA: Central nucleus of the amygdala; DMH: Dorsomedial hypothalamus; dLS: Dorsal lateral septum; LH: Lateral hypothalamus; PVN: Paraventricular nucleus of the hypothalamus; SuM: Supramammillary nucleus
Several aspects of the basic science research summarized in this review remain largely unexplored, including the translational value of these preclinical findings for human stress response coordination and how we might leverage available data from mice and rats in order to develop new pharmacological approaches to treat stress‐related clinical disorders. Translation to humans is hampered by a variety of factors, including (1) the prevalent but incorrect view that because clinical signs of stress and anxiety are rarely observed in humans receiving systemic delivery of GLP‐1 receptor agonists (e.g. liraglutide, Byetta; Bode et al., 2010; O'Neil et al., 2017; Strawn et al., 2008), it follows that central GLP‐1 signalling pathways are a poor therapeutic target for stress‐related disorders and (2), the inherent difficulty of studying the function of PrRP and other neuropeptidergic systems in humans. It is possible that while peripheral delivery of GLP‐1 receptor agonists has largely beneficial cardiovascular effects in humans (Oyama & Node, 2014; Sun et al., 2015), central GLP‐1 may contribute to elevated cardiovascular risk in chronically stressed individuals. This also leaves us with the question of whether PrRP and GLP‐1 neurons are involved in neural maladaptations to chronic stress, an arguably more translationally relevant condition than the brief interoceptive and psychogenic stressors discussed in this review. There is relatively little research on how chronic stress exposure affects PrRP and GLP‐1 neurons in rodents, but we do know that (1) chronic stress induces long‐lasting activation of cNTS neurons (Flak et al., 2012), including A2 neurons, (2) chronic stress modulates GLP‐1 expression in the cNTS via glucocorticoid receptor signalling (Zhang et al., 2010) and (3) PrRP mRNA (and TH) levels increase in response to repeated restraint stress in male rats (Tóth et al., 2008). Additional focused research is needed to establish a role for both neuropeptides in the maladaptive consequences of chronic stress exposure, including cardiovascular pathologies and affective disorders.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
AUTHOR CONTRIBUTIONS
M.K.H. and L.R. drafted the manuscript. M.K.H. created the figures. M.K.H. and L.R. edited and revised the manuscript. M.K.H. and L.R. approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
ACKNOWLEDGEMENTS
This research was supported by National Institute of Mental Health (NIH) grant number MH059911 and National Institute of Diabetes and Digestive and Kidney Diseases grant number DK100685 (to L.R.).
Holt, M. K. , & Rinaman, L. (2022). The role of nucleus of the solitary tract glucagon‐like peptide‐1 and prolactin‐releasing peptide neurons in stress: anatomy, physiology and cellular interactions. British Journal of Pharmacology, 179(4), 642–658. 10.1111/bph.15576
Funding information National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: DK100685; National Institute of Mental Health, Grant/Award Number: MH059911
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Acuna‐Goycolea, C. , & van den Pol, A. (2004). Glucagon‐like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: Implications for viscera‐mediated arousal. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24, 8141–8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi, S. , Mochiduki, A. , Nemoto, H. , Sun, B. , Fujiwara, K. , Matsumoto, H. , & Inoue, K. (2005). Estrogen suppresses the stress response of prolactin‐releasing peptide‐producing cells. Neuroscience Letters, 380, 311–315. 10.1016/j.neulet.2005.01.064 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen, M. C. , Bailey, T. W. , Jin, Y.‐H. , McDougall, S. J. , Peters, J. H. , & Aicher, S. A. (2007). Cellular heterogeneity within the solitary tract nucleus and visceral afferent processing—Electrophysiological approaches to discerning pathway performance. Tzu Chi Medical Journal, 19, 181–185. [Google Scholar]
- Andresen, M. C. , & Mendelowitz, D. (1996). Sensory afferent neurotransmission in caudal nucleus tractus solitarius‐‐common denominators. Chemical Senses, 21, 387–395. [DOI] [PubMed] [Google Scholar]
- Anesten, F. , Holt, M. K. , Schele, E. , Palsdottir, V. , Reimann, F. , Gribble, F. M. , Safari, C. , Skibicka, K. P. , Trapp, S. , & Jansson, J.‐O. (2016). Preproglucagon neurons in the hindbrain have IL‐6 receptor‐alpha and show Ca2+ influx in response to IL‐6. American journal of physiology. Regulatory. Integrative and Comparative Physiology, 311, R115–R123. 10.1152/ajpregu.00383.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard, S. M. , Marks, D. , Kobayashi, K. , Okano, H. , Low, M. J. , & Andresen, M. C. (2007). Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. The Journal of Neuroscience, 27, 13292–13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio, L. L. , Ussher, J. R. , McLean, B. A. , Cao, X. , Kabir, M. G. , Mulvihill, E. E. , Mighiu, A. S. , Zhang, H. , Ludwig, A. , Seeley, R. J. , Heximer, S. P. , & Drucker, D. J. (2017). The autonomic nervous system and cardiac GLP‐1 receptors control heart rate in mice. Molecular Metabolism, 6, 1339–1349. 10.1016/j.molmet.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragán, J. M. , Eng, J. , Rodríguez, R. , & Blázquez, E. (1999). Neural contribution to the effect of glucagon‐like peptide‐1‐(7—36) amide on arterial blood pressure in rats. American Journal of Physiology Endocrinology and Metabolism, 277, E784–E791. 10.1152/ajpendo.1999.277.5.E784 [DOI] [PubMed] [Google Scholar]
- Bechtold, D. A. , & Luckman, S. M. (2006). Prolactin‐releasing peptide mediates cholecystokinin‐induced satiety in mice. Endocrinology, 147, 4723–4729. 10.1210/en.2006-0753 [DOI] [PubMed] [Google Scholar]
- Beiroa, D. , Imbernon, M. , Gallego, R. , Senra, A. , Herranz, D. , Villarroya, F. , Serrano, M. , Ferno, J. , Salvador, J. , Escalada, J. , Dieguez, C. , Lopez, M. , Fruhbeck, G. , & Nogueiras, R. (2014). GLP‐1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes, 63, 3346–3358. 10.2337/db14-0302 [DOI] [PubMed] [Google Scholar]
- Bienkowski, M. S. , & Rinaman, L. (2008). Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic‐pituitary‐adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience, 156, 1093–1102. 10.1016/j.neuroscience.2008.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski, M. S. , & Rinaman, L. (2011). Immune challenge activates neural inputs to the ventrolateral bed nucleus of the stria terminalis. Physiology & Behavior, 104, 257–265. 10.1016/j.physbeh.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode, B.W. , Testa, M.A. , Magwire, M. , Hale, P.M. , Hammer, M. , Blonde, L. , Garber A., & on behalf of the LEAD‐3 Study Group . (2010). Patient‐reported outcomes following treatment with the human GLP‐1 analogue liraglutide or glimepiride in monotherapy: Results from a randomized controlled trial in patients with type 2 diabetes. Diabetes, Obesity & Metabolism 12: 604–612, DOI: 10.1111/j.1463-1326.2010.01196.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner, T. , Liberini, C. G. , Lutz, T. A. , & Riediger, T. (2018). Brainstem GLP‐1 signalling contributes to cancer anorexia‐cachexia syndrome in the rat. Neuropharmacology, 131, 282–290. 10.1016/j.neuropharm.2017.12.024 [DOI] [PubMed] [Google Scholar]
- Brierley, D. I. , Holt, M. K. , Singh, A. , de Araujo, A. , McDougle, M. , Vergara, M. , Afaghani, M. H. , Lee, S. J. , Scott, K. , Maske, C. , Langhans, W. , Krause, E. , de Kloet, A. , Gribble, F. M. , Reimann, F. , Rinaman, L. , de Lartigue, G. , & Trapp, S. (2021). Central and peripheral GLP‐1 systems independently suppress eating. Nature Metabolism, 3, 258–273. 10.1038/s42255-021-00344-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard, D. L. , Lynn, R. B. , Wiedner, E. B. , & Altschuler, S. M. (1998). Solitarial premotor neuron projections to the rat esophagus and pharynx: Implications for control of swallowing. Gastroenterology, 114, 1268–1275. 10.1016/S0016-5085(98)70433-0 [DOI] [PubMed] [Google Scholar]
- Card, J. P. , Johnson, A. L. , Llewellyn‐Smith, I. J. , Zheng, H. , Anand, R. , Brierley, D. I. , Trapp, S. , & Rinaman, L. (2018). GLP‐1 neurons form a local synaptic circuit within the rodent nucleus of the solitary tract. The Journal of Comparative Neurology, 526, 2149–2164. 10.1002/cne.24482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, W. , Ndoka, E. , Hutch, C. R. , Roelofs, K. , Mackinnon, A. , Khoury, B. , Magrisso, J. , Kim, K. S. , Rhodes, C. J. , Olson, D. P. , Seeley, R. J. , Sandoval, D. , & Myers, M. G. Jr. (2020). Leptin receptor‐expressing nucleus Tractus Solitarius neurons suppress food intake independently of GLP1 in mice. JCI Insight, 5, e134359. 10.1172/jci.insight.134359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork, S. C. , Richards, J. E. , Holt, M. K. , Gribble, F. M. , Reimann, F. , & Trapp, S. (2015). Distribution and characterisation of glucagon‐like peptide‐1 receptor expressing cells in the mouse brain. Molecular Metabolism, 4, 718–731. 10.1016/j.molmet.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, X. S. , & Grill, H. J. (2018). The hindbrain is a site of energy balance action for prolactin‐releasing peptide: Feeding and thermic effects from GPR10 stimulation of the nucleus tractus solitarius/area postrema. Psychopharmacology, 235, 2287–2301. 10.1007/s00213-018-4925-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas, C. V. , Buller, K. M. , Crane, J. W. , Xu, Y. , & Day, T. A. (2001). Stressor categorization: Acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. The European Journal of Neuroscience, 14, 1143–1152. 10.1046/j.0953-816x.2001.01733.x [DOI] [PubMed] [Google Scholar]
- Dayas, C. V. , Buller, K. M. , & Day, T. A. (2001). Medullary neurones regulate hypothalamic corticotropin‐releasing factor cell responses to an emotional stressor. Neuroscience, 105, 707–719. 10.1016/S0306-4522(01)00213-5 [DOI] [PubMed] [Google Scholar]
- Dayas, C. V. , Buller, K. M. , & Day, T. A. (2004). Hypothalamic paraventricular nucleus neurons regulate medullary catecholamine cell responses to restraint stress. The Journal of Comparative Neurology, 478, 22–34. 10.1002/cne.20259 [DOI] [PubMed] [Google Scholar]
- Dodd, G. T. , & Luckman, S. M. (2013). Physiological roles of GPR10 and PrRP signaling. Frontiers in Endocrinology (Lausanne), 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd, G. T. , Worth, A. A. , Nunn, N. , Korpal, A. K. , Bechtold, D. A. , Allison, M. B. , Myers, M. G. Jr. , Statnick, M. A. , & Luckman, S. M. (2014). The Thermogenic effect of Leptin is dependent on a distinct population of prolactin‐releasing peptide neurons in the Dorsomedial hypothalamus. Cell Metabolism, 20, 639–649. 10.1016/j.cmet.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun, N. J. , Dun, S. L. , & Chiaia, N. L. (1993). Hemorrhage induces Fos immunoreactivity in rat medullary catecholaminergic neurons. Brain Research, 608, 223–232. 10.1016/0006-8993(93)91462-2 [DOI] [PubMed] [Google Scholar]
- Edwards, C.M. , Dolezel, T. , & Rinaman, L. (2021). Sex and metabolic state interact to influence expression of passive avoidance memory in rats: Potential contribution of A2 noradrenergic neurons. BioRxiv 2021.02.04.429639. [DOI] [PMC free article] [PubMed]
- Ellacott, K. L. J. , Lawrence, C. B. , Pritchard, L. E. , & Luckman, S. M. (2003). Repeated administration of the anorectic factor prolactin‐releasing peptide leads to tolerance to its effects on energy homeostasis. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 285, R1005–R1010. 10.1152/ajpregu.00237.2003 [DOI] [PubMed] [Google Scholar]
- Ellacott, K. L. J. , Lawrence, C. B. , Rothwell, N. J. , & Luckman, S. M. (2002). PRL‐releasing peptide interacts with leptin to reduce food intake and body weight. Endocrinology, 143, 368–374. 10.1210/endo.143.2.8608 [DOI] [PubMed] [Google Scholar]
- Farkas, E. , Szilvásy‐Szabó, A. , Ruska, Y. , Sinkó, R. , Rasch, M. G. , Egebjerg, T. , Pyke, C. , Gereben, B. , Knudsen, L. B. , & Fekete, C. (2020). Distribution and ultrastructural localization of the glucagon‐like peptide‐1 receptor (GLP‐1R) in the rat brain. Brain Structure & Function, 226, 225–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak, J. N. , Solomon, M. B. , Jankord, R. , Krause, E. G. , & Herman, J. P. (2012). Identification of chronic stress activated regions reveals a potential recruited circuit in rat brain. The European Journal of Neuroscience, 36, 2547–2555. 10.1111/j.1460-9568.2012.08161.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin, S. M. , Chartoff, E. H. , & Roitman, M. F. (2016). The aversive agent lithium chloride suppresses phasic dopamine release through central GLP‐1 receptors. Neuropsychopharmacology, 41, 906–915. 10.1038/npp.2015.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong, T. M. , McDowall, L. M. , Horiuchi, J. , Polson, J. W. , & Dampney, R. A. L. (2014). The effect of air puff stress on c‐Fos expression in rat hypothalamus and brainstem: Central circuitry mediating sympathoexcitation and baroreflex resetting. The European Journal of Neuroscience, 39, 1429–1438. 10.1111/ejn.12521 [DOI] [PubMed] [Google Scholar]
- Gabery, S. , Salinas, C. G. , Paulsen, S. J. , Ahnfelt‐Rønne, J. , Alanentalo, T. , Baquero, A. F. , Buckley, S. T. , Farkas, E. , Fekete, C. , Frederiksen, K. S. , Helms, H. C. C. , Jeppesen, J. F. , John, L. M. , Pyke, C. , Nøhr, J. , Lu, T. T. , Polex‐Wolf, J. , Prevot, V. , Raun, K. , … Hogendorf, W. F. J. (2020). Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight, 5, e133429. 10.1172/jci.insight.133429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema, R. P. , Newmyer, B. A. , Ottolini, M. , Raje, V. , Warthen, D. M. , Lambeth, P. S. , Niccum, M. , Yao, T. , Huang, Y. , Schulman, I. G. , Harris, T. E. , Patel, M. K. , Williams, K. W. , & Scott, M. M. (2017). Activation of murine pre‐proglucagon‐producing neurons reduces food intake and body weight. The Journal of Clinical Investigation, 127, 1031–1045. 10.1172/JCI81335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema, R. P. A. , Daniels, T. E. , Shapiro, N. J. , Thacker, G. C. , Park, S.‐M. , & Goehler, L. E. (2009). Immune challenge and satiety‐related activation of both distinct and overlapping neuronal populations in the brainstem indicate parallel pathways for viscerosensory signaling. Brain Research, 1294, 61–79. 10.1016/j.brainres.2009.07.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal, S. , Packard, A. E. B. , Mahbod, P. , McKlveen, J. M. , Seeley, R. J. , Myers, B. , Ulrich‐Lai, Y. , Smith, E. P. , D'Alessio, D. A. , & Herman, J. P. (2017). Disruption of glucagon‐like peptide 1 signaling in Sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. The Journal of Neuroscience, 37, 184–193. 10.1523/JNEUROSCI.1104-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil‐Lozano, M. , Perez‐Tilve, D. , Alvarez‐Crespo, M. , Martis, A. , Fernandez, A. M. , Catalina, P. A. , Gonzalez‐Matias, L. C. , & Mallo, F. (2010). GLP‐1(7‐36)‐amide and Exendin‐4 stimulate the HPA axis in rodents and humans. Endocrinology, 151, 2629–2640. 10.1210/en.2009-0915 [DOI] [PubMed] [Google Scholar]
- Gribble, F. M. , & Reimann, F. (2021). Metabolic messengers: Glucagon‐like peptide 1. Nature Metabolism, 3, 142–148. 10.1038/s42255-020-00327-x [DOI] [PubMed] [Google Scholar]
- Griffioen, K. J. , Wan, R. , Okun, E. , Wang, X. , Lovett‐Barr, M. R. , Li, Y. , Mughal, M. R. , Mendelowitz, D. , & Mattson, M. P. (2011). GLP‐1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovascular Research, 89, 72–78. 10.1093/cvr/cvq271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill, H. J. (2020). A role for GLP‐1 in treating Hyperphagia and obesity. Endocrinology, 161, bqaa093. 10.1210/endocr/bqaa093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill, H. J. , Carmody, J. S. , Amanda Sadacca, L. , Williams, D. L. , & Kaplan, J. M. (2004). Attenuation of lipopolysaccharide anorexia by antagonism of caudal brain stem but not forebrain GLP‐1‐R. American Journal of Physiology ‐ Regulatory, Integrative and Comparative Physiology, 287, R1190–R1193. 10.1152/ajpregu.00163.2004 [DOI] [PubMed] [Google Scholar]
- Grill, H. J. , & Hayes, M. R. (2009). The nucleus tractus solitarius: A portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. International Journal of Obesity, 33(Suppl 1), S11–S15. 10.1038/ijo.2009.10 [DOI] [PubMed] [Google Scholar]
- Gu, G. , Roland, B. , Tomaselli, K. , Dolman, C. S. , Lowe, C. , & Heilig, J. S. (2013). Glucagon‐like peptide‐1 in the rat brain: Distribution of expression and functional implication. The Journal of Comparative Neurology, 521, 2235–2261. 10.1002/cne.23282 [DOI] [PubMed] [Google Scholar]
- Hinuma, S. , Habata, Y. , Fujii, R. , Kawamata, Y. , Hosoya, M. , Fukusumi, S. , Kitada, C. , Masuo, Y. , Asano, T. , Matsumoto, H. , Sekiguchi, M. , Kurokawa, T. , Nishimura, O. , Onda, H. , & Fujino, M. (1998). A prolactin‐releasing peptide in the brain. Nature, 393, 272–276. 10.1038/30515 [DOI] [PubMed] [Google Scholar]
- Hisadome, K. , Reimann, F. , Gribble, F. M. , & Trapp, S. (2010). Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: Electrical properties of glucagon‐like peptide 1 neurons. Diabetes, 59, 1890–1898. 10.2337/db10-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisadome, K. , Reimann, F. , Gribble, F. M. , & Trapp, S. (2011). CCK stimulation of GLP‐1 neurons involves α1‐adrenoceptor‐mediated increase in glutamatergic synaptic inputs. Diabetes, 60, 2701–2709. 10.2337/db11-0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, J. M. , Anekonda, V. T. , Thompson, B. W. , Zhu, M. , Curry, R. W. , Hwang, B. H. , Morton, G. J. , Schwartz, M. W. , Baskin, D. G. , Appleyard, S. M. , & Blevins, J. E. (2014). Hindbrain oxytocin receptors contribute to the effects of circulating oxytocin on food intake in male rats. Endocrinology, 155, 2845–2857. 10.1210/en.2014-1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, M. K. , Cook, D. R. , Brierley, D. I. , Richards, J. E. , Reimann, F. , Gourine, A. V. , Marina, N. , & Trapp, S. (2020). PPG neurons in the nucleus of the solitary tract modulate heart rate but do not mediate GLP‐1 receptor agonist‐induced tachycardia in mice. Mol Metab, 39, 101024. 10.1016/j.molmet.2020.101024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, M. K. , Llewellyn‐Smith, I. J. , Reimann, F. , Gribble, F. M. , & Trapp, S. (2017). Serotonergic modulation of the activity of GLP‐1 producing neurons in the nucleus of the solitary tract in mouse. Mol Metab, 6, 909–921. 10.1016/j.molmet.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, M. K. , Pomeranz, L. E. , Beier, K. T. , Reimann, F. , Gribble, F. M. , & Rinaman, L. (2019). Synaptic inputs to the mouse dorsal vagal complex and its resident preproglucagon neurons. The Journal of Neuroscience, 39, 9767–9781. 10.1523/JNEUROSCI.2145-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, M. K. , Richards, J. E. , Cook, D. R. , Brierley, D. I. , Williams, D. L. , Reimann, F. , Gribble, F. M. , & Trapp, S. (2019). Preproglucagon neurons in the nucleus of the solitary tract are the Main source of brain GLP‐1, mediate stress‐induced Hypophagia, and limit unusually large intakes of food. Diabetes, 68, 21–33. 10.2337/db18-0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, M. K. , & Trapp, S. (2016). The physiological role of the brain GLP‐1 system in stress. Cogent Biology, 2, 1229086. 10.1080/23312025.2016.1229086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi, J. , Saigusa, T. , Sugiyama, N. , Kanba, S. , Nishida, Y. , Sato, Y. , Hinuma, S. , & Arita, J. (2002). Effects of prolactin‐releasing peptide microinjection into the ventrolateral medulla on arterial pressure and sympathetic activity in rats. Brain Research, 958, 201–209. 10.1016/S0006-8993(02)03718-6 [DOI] [PubMed] [Google Scholar]
- Hsu, T. M. , Hahn, J. D. , Konanur, V. R. , Lam, A. , & Kanoski, S. E. (2015). Hippocampal GLP‐1 receptors influence food intake, meal size, and effort‐based responding for food through volume transmission. Neuropsychopharmacology, 40, 327–337. 10.1038/npp.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo, L. , Gamber, K. M. , Grill, H. J. , & Bjørbaek, C. (2008). Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology, 149, 492–497. 10.1210/en.2007-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima, N. , Kataoka, Y. , Kakihara, K. , Bamba, H. , Tamada, Y. , Hayashi, S. , Matsuda, T. , Tanaka, M. , Honjyo, H. , Hosoya, M. , Hinuma, S. , & Ibata, Y. (1999). Cytochemical study of prolactin‐releasing peptide (PrRP) in the rat brain. Neuroreport, 10, 1713–1716. 10.1097/00001756-199906030-00016 [DOI] [PubMed] [Google Scholar]
- Jensen, C. B. , Pyke, C. , Rasch, M. G. , Dahl, A. B. , Knudsen, L. B. , & Secher, A. (2018). Characterization of the Glucagonlike Peptide‐1 receptor in male mouse brain using a novel antibody and in situ hybridization. Endocrinology, 159, 665–675. 10.1210/en.2017-00812 [DOI] [PubMed] [Google Scholar]
- Kalliomäki, M.‐L. , Pertovaara, A. , Brandt, A. , Wei, H. , Pietilä, P. , Kalmari, J. , Xu, M. , Kalso, E. , & Panula, P. (2004). Prolactin‐releasing peptide affects pain, allodynia and autonomic reflexes through medullary mechanisms. Neuropharmacology, 46, 412–424. 10.1016/j.neuropharm.2003.09.021 [DOI] [PubMed] [Google Scholar]
- Kamilaris, T. C. , Johnson, E. O. , Calogero, A. E. , Kalogeras, K. T. , Bernardini, R. , Chrousos, G. P. , & Gold, P. W. (1992). Cholecystokinin‐octapeptide stimulates hypothalamic‐pituitary‐adrenal function in rats: Role of corticotropin‐releasing hormone. Endocrinology, 130, 1764–1774. 10.1210/endo.130.4.1312423 [DOI] [PubMed] [Google Scholar]
- Kinzig, K. P. , D'Alessio, D. A. , Herman, J. P. , Sakai, R. R. , Vahl, T. P. , Figueiredo, H. F. , Murphy, E. K. , & Seeley, R. J. (2003). CNS glucagon‐like peptide‐1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23, 6163–6170. 10.1523/JNEUROSCI.23-15-06163.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig, K. P. , D'Alessio, D. A. , & Seeley, R. J. (2002). The diverse roles of specific GLP‐1 receptors in the control of food intake and the response to visceral illness. The Journal of Neuroscience, 22, 10470–10476. 10.1523/JNEUROSCI.22-23-10470.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisler, A. D. , Davis, E. A. , & Rinaman, L. (2014). Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non‐entrained rats after intake of satiating vs. non‐satiating meals. Physiology & Behavior, 136, 47–54. 10.1016/j.physbeh.2014.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisler, A. D. , & Rinaman, L. (2016). Hindbrain glucagon‐like peptide‐1 neurons track intake volume and contribute to injection stress‐induced hypophagia in meal‐entrained rats. American journal of physiology. Regulatory, Integrative and Comparative Physiology, 310, R906–R916. 10.1152/ajpregu.00243.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachey, J. L. , D'Alessio, D. A. , Rinaman, L. , Elmquist, J. K. , Drucker, D. J. , & Seeley, R. J. (2005). The role of central glucagon‐like peptide‐1 in mediating the effects of visceral illness: Differential effects in rats and mice. Endocrinology, 146, 458–462. 10.1210/en.2004-0419 [DOI] [PubMed] [Google Scholar]
- Larsen, P. J. , Tang‐Christensen, M. , & Jessop, D. S. (1997). Central administration of glucagon‐like peptide‐1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology, 138, 4445–4455. 10.1210/endo.138.10.5270 [DOI] [PubMed] [Google Scholar]
- Laurent, P. , Becker, J. A. J. , Valverde, O. , Ledent, C. , de Kerchove d'Exaerde, A. , Schiffmann, S. N. , Maldonado, R. , Vassart, G. , & Parmentier, M. (2005). The prolactin‐releasing peptide antagonizes the opioid system through its receptor GPR10. Nature Neuroscience, 8, 1735–1741. 10.1038/nn1585 [DOI] [PubMed] [Google Scholar]
- Lawrence, C. B. , Celsi, F. , Brennand, J. , & Luckman, S. M. (2000). Alternative role for prolactin‐releasing peptide in the regulation of food intake. Nature Neuroscience, 3, 645–646. 10.1038/76597 [DOI] [PubMed] [Google Scholar]
- Lawrence, C. B. , Ellacott, K. L. J. , & Luckman, S. M. (2002). PRL‐releasing peptide reduces food intake and may mediate satiety signaling. Endocrinology, 143, 360–367. 10.1210/endo.143.2.8609 [DOI] [PubMed] [Google Scholar]
- Lawrence, C. B. , Liu, Y.‐L. , Stock, M. J. , & Luckman, S. M. (2004). Anorectic actions of prolactin‐releasing peptide are mediated by corticotropin‐releasing hormone receptors. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 286, R101–R107. 10.1152/ajpregu.00402.2003 [DOI] [PubMed] [Google Scholar]
- Lee, S. J. , Sanchez‐Watts, G. , Krieger, J.‐P. , Pignalosa, A. , Norell, P. N. , Cortella, A. , Pettersen, K. G. , Vrdoljak, D. , Hayes, M. R. , Kanoski, S. E. , Langhans, W. , & Watts, A. G. (2018). Loss of dorsomedial hypothalamic GLP‐1 signaling reduces BAT thermogenesis and increases adiposity. Molecular Metabolism, 11, 33–46. 10.1016/j.molmet.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Navarrete, J. , Liang‐Guallpa, J. , Lu, C. , Funderburk, S. C. , Chang, R. B. , Liberles, S. D. , Olson, D. P. , & Krashes, M. J. (2019). Defined Paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metabolism, 29, 681–694.e5. 10.1016/j.cmet.2018.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. Y. , Ericsson, A. , & Sawchenko, P. E. (1996). Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proceedings of the National Academy of Sciences of the United States of America, 93, 2359–2364. 10.1073/pnas.93.6.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Conde, K. , Zhang, P. , Lilascharoen, V. , Xu, Z. , Lim, B. K. , Seeley, R. J. , Zhu, J. J. , Scott, M. M. , & Pang, Z. P. (2017). Enhanced AMPA receptor trafficking mediates the Anorexigenic effect of endogenous glucagon‐like Peptide‐1 in the Paraventricular hypothalamus. Neuron, 96, 897–909.e5. 10.1016/j.neuron.2017.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn‐Smith, I. J. , Gnanamanickam, G. J. , Reimann, F. , Gribble, F. M. , & Trapp, S. (2013). Preproglucagon (PPG) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience, 229, 130–143. 10.1016/j.neuroscience.2012.09.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn‐Smith, I. J. , Marina, N. , Manton, R. N. , Reimann, F. , Gribble, F. M. , & Trapp, S. (2015). Spinally projecting preproglucagon axons preferentially innervate sympathetic preganglionic neurons. Neuroscience, 284, 872–887. 10.1016/j.neuroscience.2014.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn‐Smith, I. J. , Reimann, F. , Gribble, F. M. , & Trapp, S. (2011). Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience, 180, 111–121. 10.1016/j.neuroscience.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockie, S. H. , Heppner, K. M. , Chaudhary, N. , Chabenne, J. R. , Morgan, D. A. , Veyrat‐Durebex, C. , Ananthakrishnan, G. , Rohner‐Jeanrenaud, F. , Drucker, D. J. , DiMarchi, R. , Rahmouni, K. , Oldfield, B. J. , Tschop, M. H. , & Perez‐Tilve, D. (2012). Direct control of brown adipose tissue thermogenesis by central nervous system glucagon‐like peptide‐1 receptor signaling. Diabetes, 61, 2753–2762. 10.2337/db11-1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Ferreras, L. , Eerola, K. , Shevchouk, O. T. , Richard, J. E. , Nilsson, F. H. , Jansson, L. E. , Jansson, E. , Hayes, M. R. , & Skibicka, K. P. (2020). The supramammillary nucleus controls anxiety‐like behavior; key role of GLP‐1R. Psychoneuroendocrinology, 119, 104720. 10.1016/j.psyneuen.2020.104720 [DOI] [PubMed] [Google Scholar]
- Ma, L. , MacTavish, D. , Simonin, F. , Bourguignon, J.‐J. , Watanabe, T. , & Jhamandas, J. H. (2009). Prolactin‐releasing peptide effects in the rat brain are mediated through the neuropeptide FF receptor. European Journal of Neuroscience, 30, 1585–1593. 10.1111/j.1460-9568.2009.06956.x [DOI] [PubMed] [Google Scholar]
- Maclusky, N. J. , Cook, S. , Scrocchi, L. , Shin, J. , Kim, J. , Vaccarino, F. , Asa, S. L. , & Drucker, D. J. (2000). Neuroendocrine function and response to stress in mice with complete disruption of glucagon‐like Peptide‐1 receptor signaling. Endocrinology, 141(11), 752–762. 10.1210/endo.141.2.7326 [DOI] [PubMed] [Google Scholar]
- Maley, B. E. (1996). Immunohistochemical localization of neuropeptides and neurotransmitters in the nucleus solitarius. Chemical Senses, 21, 367–376. 10.1093/chemse/21.3.367 [DOI] [PubMed] [Google Scholar]
- Maniscalco, J. W. , Edwards, C. M. , & Rinaman, L. (2020). Ghrelin signaling contributes to fasting‐induced attenuation of hindbrain neural activation and hypophagic responses to systemic cholecystokinin in rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 318, R1014–R1023. 10.1152/ajpregu.00346.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco, J. W. , Kreisler, A. D. , & Rinaman, L. (2012). Satiation and stress‐induced hypophagia: Examining the role of hindbrain neurons expressing prolactin‐releasing peptide or glucagon‐like peptide 1. Frontiers in Neuroscience, 6, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco, J. W. , & Rinaman, L. (2013). Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon‐like peptide‐1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiology & Behavior, 121, 35–42. 10.1016/j.physbeh.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco, J. W. , & Rinaman, L. (2014). Systemic leptin dose‐dependently increases STAT3 phosphorylation within hypothalamic and hindbrain nuclei. American Journal of Physiology ‐ Regulatory, Integrative and Comparative Physiology, 306, R576–R585. 10.1152/ajpregu.00017.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco, J. W. , & Rinaman, L. (2017). Interoceptive modulation of neuroendocrine, emotional, and hypophagic responses to stress. Physiology & Behavior, 176, 195–206. 10.1016/j.physbeh.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco, J. W. , & Rinaman, L. (2018). Vagal interoceptive modulation of motivated behavior. Physiology (Bethesda), 33, 151–167. 10.1152/physiol.00036.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco, J. W. , Zheng, H. , Gordon, P. J. , & Rinaman, L. (2015). Negative energy balance blocks neural and behavioral responses to acute stress by ‘silencing’ central glucagon‐like peptide 1 signaling in rats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35, 10701–10714. 10.1523/JNEUROSCI.3464-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama, M. , Matsumoto, H. , Fujiwara, K. , Kitada, C. , Hinuma, S. , Onda, H. , Fujino, M. , & Inoue, K. (1999). Immunocytochemical localization of prolactin‐releasing peptide in the rat brain. Endocrinology, 140, 2326–2333. 10.1210/endo.140.5.6685 [DOI] [PubMed] [Google Scholar]
- Maruyama, M. , Matsumoto, H. , Fujiwara, K. , Noguchi, J. , Kitada, C. , Fujino, M. , & Inoue, K. (2001). Prolactin‐releasing peptide as a novel stress mediator in the central nervous system. Endocrinology, 142, 2032–2038. 10.1210/endo.142.5.8118 [DOI] [PubMed] [Google Scholar]
- Matsumoto, H. , Maruyama, M. , Noguchi, J. , Horikoshi, Y. , Fujiwara, K. , Kitada, C. , Hinuma, S. , Onda, H. , Nishimura, O. , Inoue, K. , & Fujino, M. (2000). Stimulation of corticotropin‐releasing hormone‐mediated adrenocorticotropin secretion by central administration of prolactin‐releasing peptide in rats. Neuroscience Letters, 285, 234–238. 10.1016/S0304-3940(00)01077-6 [DOI] [PubMed] [Google Scholar]
- McKlveen, J. M. , Myers, B. , & Herman, J. P. (2015). The medial prefrontal cortex: Coordinator of autonomic, neuroendocrine, and behavioral responses to stress. Journal of Neuroendocrinology, 27, 446–456. 10.1111/jne.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mera, T. , Fujihara, H. , Kawasaki, M. , Hashimoto, H. , Saito, T. , Shibata, M. , Saito, J. , Oka, T. , Tsuji, S. , Onaka, T. , & Ueta, Y. (2006). Prolactin‐releasing peptide is a potent mediator of stress responses in the brain hrough the hypothalamic paraventricular nucleus. Neuroscience, 141, 1069–1086. 10.1016/j.neuroscience.2006.04.023 [DOI] [PubMed] [Google Scholar]
- Mera, T. , Fujihara, H. , Saito, J. , Kawasaki, M. , Hashimoto, H. , Saito, T. , Shibata, M. , Onaka, T. , Tanaka, Y. , Oka, T. , Tsuji, S. , & Ueta, Y. (2007). Downregulation of prolactin‐releasing peptide gene expression in the hypothalamus and brainstem of diabetic rats. Peptides, 28, 1596–1604. 10.1016/j.peptides.2007.06.023 [DOI] [PubMed] [Google Scholar]
- Merchenthaler, I. , Lane, M. , & Shughrue, P. (1999). Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. The Journal of Comparative Neurology, 403, 261–280. [DOI] [PubMed] [Google Scholar]
- Mochiduki, A. , Takeda, T. , Kaga, S. , & Inoue, K. (2010). Stress response of prolactin‐releasing peptide knockout mice as to glucocorticoid secretion. Journal of Neuroendocrinology, 22, 576–584. 10.1111/j.1365-2826.2010.01993.x [DOI] [PubMed] [Google Scholar]
- Morales, T. , Hinuma, S. , & Sawchenko, P. E. (2000). Prolactin‐releasing peptide is expressed in afferents to the endocrine hypothalamus, but not in neurosecretory neurones. Journal of Neuroendocrinology, 12, 131–140. 10.1046/j.1365-2826.2000.00428.x [DOI] [PubMed] [Google Scholar]
- Morales, T. , & Sawchenko, P. E. (2003). Brainstem prolactin‐releasing peptide neurons are sensitive to stress and lactation. Neuroscience, 121, 771–778. 10.1016/S0306-4522(03)00522-0 [DOI] [PubMed] [Google Scholar]
- Mtui, E. P. , Anwar, M. , Gomez, R. , Reis, D. J. , & Ruggiero, D. A. (1993). Projections from the nucleus tractus solitarii to the spinal cord. The Journal of Comparative Neurology, 337, 231–252. 10.1002/cne.903370205 [DOI] [PubMed] [Google Scholar]
- Navarro I Batista, K. , Schraner, M. , & Riediger, T. (2020). Brainstem prolactin‐releasing peptide contributes to cancer anorexia‐cachexia syndrome in rats. Neuropharmacology, 180, 108289. 10.1016/j.neuropharm.2020.108289 [DOI] [PubMed] [Google Scholar]
- O'Neil, P.M. , Aroda, V.R. , Astrup, A. , Kushner, R. , Lau, D.C.W. , Wadden, T.A. , Brett J., Cancino, A. P. , Wilding J. P. H., & on behalf of the Satiety and Clinical Adiposity ‐ Liraglutide Evidence in individuals with and without diabetes (SCALE) study groups . (2017). Neuropsychiatric safety with liraglutide 3.0 mg for weight management: Results from randomized controlled phase 2 and 3a trials. Diabetes, Obesity & Metabolism 19: 1529–1536, DOI: 10.1111/dom.12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama, J.‐I. , & Node, K. (2014). Incretin therapy and heart failure. Circulation Journal, 78, 819–824. 10.1253/circj.CJ-13-1561 [DOI] [PubMed] [Google Scholar]
- Palkovits, M. , Baffi, J. S. , & Pacak, K. (1997). Stress‐induced Fos‐like Immunoreactivity in the pons and the medulla oblongata of rats. Stress, 1, 155–168. 10.3109/10253899709001105 [DOI] [PubMed] [Google Scholar]
- Riche, D. , De Pommery, J. , & Menetrey, D. (1990). Neuropeptides and catecholamines in efferent projections of the nuclei of the solitary tract in the rat. The Journal of Comparative Neurology, 293, 399–424. 10.1002/cne.902930306 [DOI] [PubMed] [Google Scholar]
- Rinaman, L. (1999a). A functional role for central glucagon‐like peptide‐1 receptors in lithium chloride‐induced anorexia. The American Journal of Physiology, 277, R1537–R1540. 10.1152/ajpregu.1999.277.5.R1537 [DOI] [PubMed] [Google Scholar]
- Rinaman, L. (1999b). Interoceptive stress activates glucagon‐like peptide‐1 neurons that project to the hypothalamus. The American Journal of Physiology, 277, R582–R590. 10.1152/ajpregu.1999.277.2.R582 [DOI] [PubMed] [Google Scholar]
- Rinaman, L. (2003). Hindbrain noradrenergic lesions attenuate anorexia and Alter central cFos expression in rats after gastric Viscerosensory stimulation. The Journal of Neuroscience, 23, 10084–10092. 10.1523/JNEUROSCI.23-31-10084.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman, L. (2007). Visceral sensory inputs to the endocrine hypothalamus. Frontiers in Neuroendocrinology, 28, 50–60. 10.1016/j.yfrne.2007.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman, L. (2010). Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Research, 1350, 18–34. 10.1016/j.brainres.2010.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman, L. , Card, J. P. , Schwaber, J. S. , & Miselis, R. R. (1989). Ultrastructural demonstration of a gastric monosynaptic vagal circuit in the nucleus of the solitary tract in rat. The Journal of Neuroscience, 9, 1985–1996. 10.1523/JNEUROSCI.09-06-01985.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman, L. , & Dzmura, V. (2007). Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 293, R1495–R1503. 10.1152/ajpregu.00393.2007 [DOI] [PubMed] [Google Scholar]
- Rinaman, L. , Hoffman, G. E. , Dohanics, J. , Le, W. W. , Stricker, E. M. , & Verbalis, J. G. (1995). Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. The Journal of Comparative Neurology, 360, 246–256. 10.1002/cne.903600204 [DOI] [PubMed] [Google Scholar]
- Roland, B. L. , Sutton, S. W. , Wilson, S. J. , Luo, L. , Pyati, J. , Huvar, R. , Erlander, M. G. , & Lovenberg, T. W. (1999). Anatomical distribution of prolactin‐releasing peptide and its receptor suggests additional functions in the central nervous system and periphery. Endocrinology, 140, 5736–5745. 10.1210/endo.140.12.7211 [DOI] [PubMed] [Google Scholar]
- Saha, S. , Batten, T. F. , & Henderson, Z. (2000). A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: A combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience, 99, 613–626. 10.1016/S0306-4522(00)00240-2 [DOI] [PubMed] [Google Scholar]
- Samson, W. K. , Resch, Z. T. , & Murphy, T. C. (2000). A novel action of the newly described prolactin‐releasing peptides: Cardiovascular regulation. Brain Research, 858, 19–25. 10.1016/S0006-8993(99)02451-8 [DOI] [PubMed] [Google Scholar]
- Sarkar, S. , Fekete, C. , Legradi, G. , & Lechan, R. M. (2003). Glucagon like peptide‐1 (7‐36) amide (GLP‐1) nerve terminals densely innervate corticotropin‐releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Research, 985, 163–168. 10.1016/S0006-8993(03)03117-2 [DOI] [PubMed] [Google Scholar]
- Sawchenko, P. E. , Arias, C. , & Bittencourt, J. C. (1990). Inhibin beta, somatostatin, and enkephalin immunoreactivities coexist in caudal medullary neurons that project to the paraventricular nucleus of the hypothalamus. The Journal of Comparative Neurology, 291, 269–280. 10.1002/cne.902910209 [DOI] [PubMed] [Google Scholar]
- Sawchenko, P. E. , & Swanson, L. W. (1982). The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Research, 257, 275–325. 10.1016/0165-0173(82)90010-8 [DOI] [PubMed] [Google Scholar]
- Seal, L. J. , Small, C. J. , Dhillo, W. S. , Kennedy, A. R. , Ghatei, M. A. , & Bloom, S. R. (2002). Prolactin‐releasing peptide releases Corticotropin‐releasing hormone and increases plasma Adrenocorticotropin via the Paraventricular nucleus of the hypothalamus. Nen, 76, 70–78. [DOI] [PubMed] [Google Scholar]
- Seeley, R. J. , Blake, K. , Rushing, P. A. , Benoit, S. , Eng, J. , Woods, S. C. , & D'Alessio, D. (2000). The role of CNS glucagon‐like peptide‐1 (7‐36) amide receptors in mediating the visceral illness effects of lithium chloride. The Journal of Neuroscience, 20, 1616–1621. 10.1523/JNEUROSCI.20-04-01616.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta, R. L. , Sevigny, C. P. , & Guyenet, P. G. (2002). Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. The Journal of Comparative Neurology, 444, 191–206. 10.1002/cne.10141 [DOI] [PubMed] [Google Scholar]
- Strawn, J. R. , D'Alessio, D. A. , Keck, P. E. , & Seeley, R. J. (2008). Failure of glucagon‐like peptide‐1 to induce panic attacks or anxiety in patients with panic disorder. Journal of Psychiatric Research, 42, 787–789. 10.1016/j.jpsychires.2007.08.007 [DOI] [PubMed] [Google Scholar]
- Sun, B. , Nemoto, H. , Fujiwara, K. , Adachi, S. , & Inoue, K. (2005). Nicotine stimulates prolactin‐releasing peptide (PrRP) cells and non‐PrRP cells in the solitary nucleus. Regulatory Peptides, 126, 91–96. 10.1016/j.regpep.2004.08.025 [DOI] [PubMed] [Google Scholar]
- Sun, F. , Wu, S. , Guo, S. , Yu, K. , Yang, Z. , Li, L. , Zhang, Y. , Quan, X. , Ji, L. , & Zhan, S. (2015). Impact of GLP‐1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: A systematic review and network meta‐analysis. Diabetes Research and Clinical Practice, 110, 26–37. 10.1016/j.diabres.2015.07.015 [DOI] [PubMed] [Google Scholar]
- Takayanagi, Y. , & Onaka, T. (2010). Roles of prolactin‐releasing peptide and RFamide related peptides in the control of stress and food intake. The FEBS Journal, 277, 4998–5005. 10.1111/j.1742-4658.2010.07932.x [DOI] [PubMed] [Google Scholar]
- Terrill, S. J. , Holt, M. K. , Maske, C. B. , Abrams, N. , Reimann, F. , Trapp, S. , & Williams, D. L. (2019). Endogenous GLP‐1 in lateral septum promotes satiety and suppresses motivation for food in mice. Physiology & Behavior, 206, 191–199. 10.1016/j.physbeh.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrill, S. J. , Maske, C. B. , & Williams, D. L. (2018). Endogenous GLP‐1 in lateral septum contributes to stress‐induced hypophagia. Physiology & Behavior, 192, 17–22. 10.1016/j.physbeh.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaud, N. , Gribble, F. , Reimann, F. , Trapp, S. , & Fadool, D. A. (2019). A unique olfactory bulb microcircuit driven by neurons expressing the precursor to glucagon‐like peptide 1. Scientific Reports, 9, 15542. 10.1038/s41598-019-51880-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth, Z. E. , Zelena, D. , Mergl, Z. , Kirilly, E. , Várnai, P. , Mezey, E. , Makara, G. B. , & Palkovits, M. (2008). Chronic repeated restraint stress increases prolactin‐releasing peptide/tyrosine‐hydroxylase ratio with gender‐related differences in the rat brain. Journal of Neurochemistry, 104, 653–666. 10.1111/j.1471-4159.2007.05069.x [DOI] [PubMed] [Google Scholar]
- Travagli, R. A. , Hermann, G. E. , Browning, K. N. , & Rogers, R. C. (2006). Brainstem circuits regulating gastric function. Annual Review of Physiology, 68, 279–305. 10.1146/annurev.physiol.68.040504.094635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang, A. H. , Nuzzaci, D. , Darwish, T. , Samudrala, H. , & Blouet, C. (2020). Nutrient sensing in the nucleus of the solitary tract mediates non‐aversive suppression of feeding via inhibition of AgRP neurons. Molecular Metabolism, 42, 101070. 10.1016/j.molmet.2020.101070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton, M. D. , O'Shea, D. , Gunn, I. , Beak, S. A. , Edwards, C. M. , Meeran, K. , Choi, S. J. , Taylor, G. M. , Heath, M. M. , Lambert, P. D. , Wilding, J. P. H. , Smith, D. M. , Ghatei, M. A. , Herbert, J. , & Bloom, S. R. (1996). A role for glucagon‐like peptide‐1 in the central regulation of feeding. Nature, 379, 69–72. 10.1038/379069a0 [DOI] [PubMed] [Google Scholar]
- Uchida, K. , Kobayashi, D. , Das, G. , Onaka, T. , Inoue, K. , & Itoi, K. (2010). Participation of the prolactin‐releasing peptide‐containing neurones in caudal medulla in conveying haemorrhagic stress‐induced signals to the paraventricular nucleus of the hypothalamus. Journal of Neuroendocrinology, 22, 33–42. 10.1111/j.1365-2826.2009.01935.x [DOI] [PubMed] [Google Scholar]
- Ulrich‐Lai, Y. M. , & Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews. Neuroscience, 10, 397–409. 10.1038/nrn2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol, A. N. (2012). Neuropeptide transmission in brain circuits. Neuron, 76, 98–115. 10.1016/j.neuron.2012.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooy, D. , Koda, L. Y. , McGinty, J. F. , Gerfen, C. R. , & Bloom, F. E. (1984). The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. The Journal of Comparative Neurology, 224, 1–24. 10.1002/cne.902240102 [DOI] [PubMed] [Google Scholar]
- van Dijk, G. , & Thiele, T. E. (1999). Glucagon‐like peptide‐1 (7–36) amide: A central regulator of satiety and interoceptive stress. Neuropeptides, 33, 406–414. 10.1054/npep.1999.0053 [DOI] [PubMed] [Google Scholar]
- Veening, J. G. , Swanson, L. W. , & Sawchenko, P. E. (1984). The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: A combined retrograde transport‐immunohistochemical study. Brain Research, 303, 337–357. 10.1016/0006-8993(84)91220-4 [DOI] [PubMed] [Google Scholar]
- Vrang, N. , Phifer, C. B. , Corkern, M. M. , & Berthoud, H. R. (2003). Gastric distension induces c‐Fos in medullary GLP‐1/2‐containing neurons. American journal of physiology. Regulatory. Integrative and Comparative Physiology, 285, R470–R478. 10.1152/ajpregu.00732.2002 [DOI] [PubMed] [Google Scholar]
- Wall, K. D. , Olivos, D. R. , & Rinaman, L. (2020). High fat diet attenuates cholecystokinin‐induced cFos activation of prolactin‐releasing peptide‐expressing A2 noradrenergic neurons in the caudal nucleus of the solitary tract. Neuroscience, 447, 113–121. 10.1016/j.neuroscience.2019.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D. L. , Baskin, D. G. , & Schwartz, M. W. (2009). Hindbrain leptin receptor stimulation enhances the anorexic response to cholecystokinin. American Journal of Physiology ‐ Regulatory, Integrative and Comparative Physiology, 297, R1238–R1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D. L. , Lilly, N. A. , Edwards, I. J. , Yao, P. , Richards, J. E. , & Trapp, S. (2018). GLP‐1 action in the mouse bed nucleus of the stria terminalis. Neuropharmacology, 131, 83–95. 10.1016/j.neuropharm.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, T. , Mochiduki, A. , Sugimoto, Y. , Suzuki, Y. , Itoi, K. , & Inoue, K. (2009). Prolactin‐releasing peptide regulates the cardiovascular system via corticotrophin‐releasing hormone. Journal of Neuroendocrinology, 21, 586–593. 10.1111/j.1365-2826.2009.01875.x [DOI] [PubMed] [Google Scholar]
- Yamamoto, H. , Lee, C. E. , Marcus, J. N. , Williams, T. D. , Overton, J. M. , Lopez, M. E. , Hollenberg, A. N. , Baggio, L. , Saper, C. B. , Drucker, D. J. , & Elmquist, J. K. (2002). Glucagon‐like peptide‐1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. The Journal of Clinical Investigation, 110, 43–52. 10.1172/JCI0215595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, M. , Takayanagi, Y. , & Onaka, T. (2014). The medial amygdala‐medullary PrRP‐synthesizing neuron pathway mediates neuroendocrine responses to contextual conditioned fear in male rodents. Endocrinology, 155, 2996–3004. 10.1210/en.2013-1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R. , Jankord, R. , Flak, J. N. , Solomon, M. B. , D'Alessio, D. A. , & Herman, J. P. (2010). Role of glucocorticoids in tuning hindbrain stress integration. The Journal of Neuroscience, 30, 14907–14914. 10.1523/JNEUROSCI.0522-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, H. , Reiner, D. J. , Hayes, M. R. , & Rinaman, L. (2019). Chronic suppression of glucagon‐like Peptide‐1 receptor (GLP1R) mRNA translation in the rat bed nucleus of the Stria Terminalis reduces anxiety‐like behavior and stress‐induced Hypophagia, but prolongs stress‐induced elevation of plasma Corticosterone. The Journal of Neuroscience, 39, 2649–2663. 10.1523/JNEUROSCI.2180-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, H. , Stornetta, R. L. , Agassandian, K. , & Rinaman, L. (2015). Glutamatergic phenotype of glucagon‐like peptide 1 neurons in the caudal nucleus of the solitary tract in rats. Brain Structure & Function, 220, 3011–3022. 10.1007/s00429-014-0841-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L. , & Onaka, T. (2002). Involvement of medullary A2 noradrenergic neurons in the activation of oxytocin neurons after conditioned fear stimuli. European Journal of Neuroscience, 16, 2186–2198. 10.1046/j.1460-9568.2002.02285.x [DOI] [PubMed] [Google Scholar]
- Zhu, L. L. , & Onaka, T. (2003). Facilitative role of prolactin‐releasing peptide neurons in oxytocin cell activation after conditioned‐fear stimuli. Neuroscience, 118, 1045–1053. 10.1016/S0306-4522(03)00059-9 [DOI] [PubMed] [Google Scholar]
- Zoccal, D. B. , Furuya, W. I. , Bassi, M. , Colombari, D. S. A. , & Colombari, E. (2014). The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Frontiers in Physiology, 5, 238. 10.3389/fphys.2014.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


