Fig. 6.
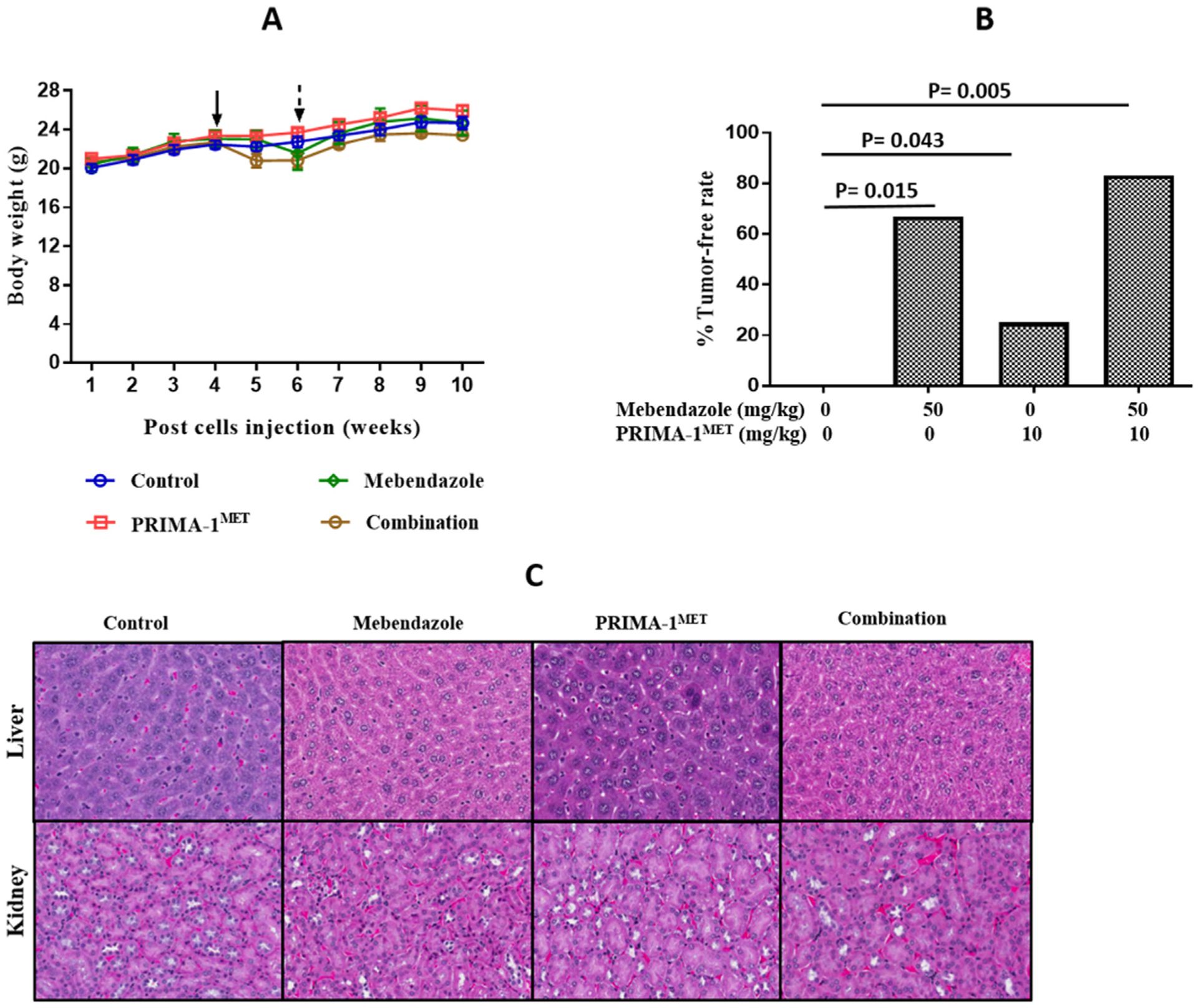
In vivo validation of effect of mebendazole alone or in combination with PRIMA-1MET on ovarian cancer treatment. Tumors were established in 6 week old athymic nude mice by i.p. injection of 2 × 107 MES-OV GFP/LUC cells. Peritoneal tumors were excised from MES-OV injected athymic nude mice after the treatment period. (A) Mouse body weight throughout the experiment. The solid arrow indicates when treatment was started and the dotted arrow indicates when the dosing schedule was reduced. (B) Tumor free rates in the treatment groups, Logistic Regression Model: Mebendazole (p = 0.005, OR = 27.535, 95% CI: 2.725, 278.265) and PRIMA-1MET (p = 0.182, OR = 4.998, 95% CI: 0.472, 52.936) functioned additively in preventing tumor development. # Fisher’s exact test demonstrated that the combination was more effective compared to control p = 0.015 and compared to PRIMA-1METp = 0.043. (C) 20× imaging of H & E staining of liver and kidney specimen of mice tumor model to determine toxicity of drug combination.
