Fig. 2 |. Mechanistic studies.
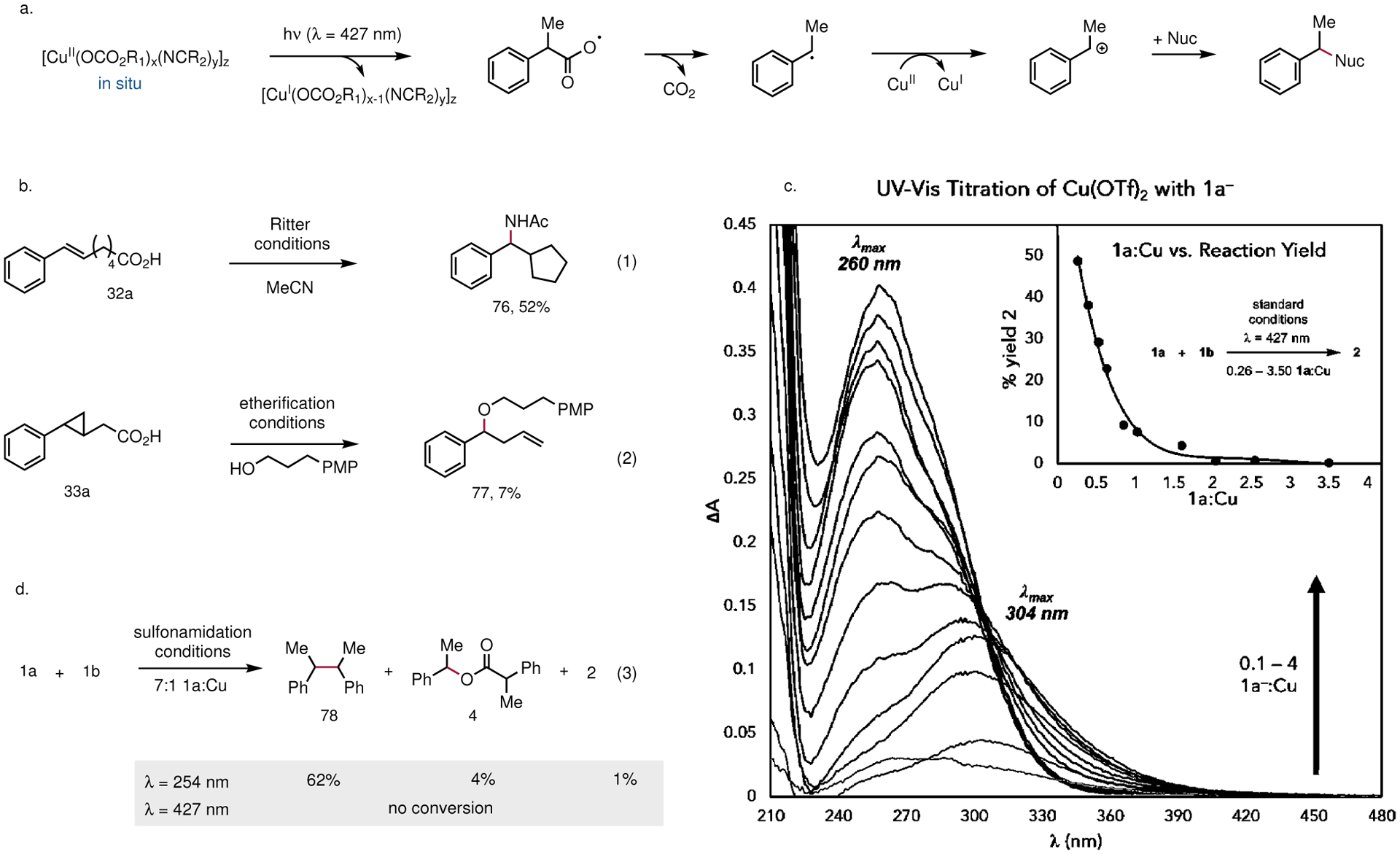
a, The guiding mechanistic hypothesis for this reaction involves MLCT photoexcitation of a preassembled Cu(II) carboxylate complex, spontaneous decarboxylation of the resulting carboxyl radical, and Cu(II)-mediated oxidative coupling of the corresponding radical. b, Decarboxylative cyclization under Ritter amidation conditions is consistent with a putative radical intermediate. c, Decarboxylative ring-opening of a radical clock substrate. d, UV-vis titration study to interrogate the interaction between the carboxylate derived from 1a and Cu(II). An initial species is formed with λmax = 304 nm at low carboxylate loadings, which transitions to a blue-shifted species (λmax = 260 nm) at high carboxylate loadings. The species formed at high carboxylate loadings is photochemically inactive at 427 nm, in line with the empirically optimized conditions. e, UV irradiation at high acid equivalents still leads to radical generation, but chemoselective formation of dimer 78 is observed in lieu of the expected product 2.
