Table 2 |.
Scope of decarboxylative cross-coupling with sulfonamide nucleophiles.
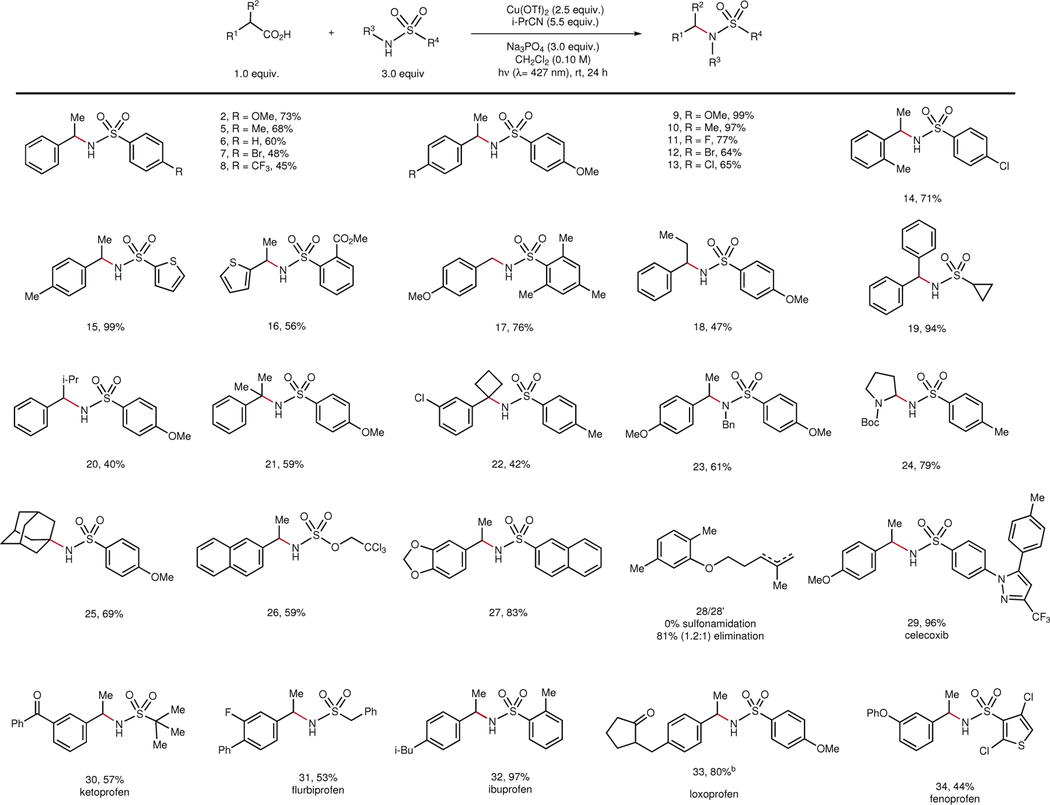
|
0.20 mmol scale isolation conditions: Cu(OTf)2 (2.5 equiv.), Na3PO4 (3.0 equiv.), sulfonamide (3.0 equiv.), carboxylic acid (1.0 equiv.), CH2Cl2 (0.10 M), and i-PrCN (5.5 equiv.) are added to a 1.5-dram reaction vial equipped with a stir bar in a glovebox. The vial is irradiated by two 34 W blue LEDs at a distance of 10 cm.
The 1.2:1 ratio refers to the trisubstited:1,1-disubstituted olefin product distribution as determined by 1H NMR of the crude reaction mixture.
Diastereomers were not detected in NMR analysis.
