Table 3 |.
Scope of decarboxylative cross-coupling with different classes of nucleophiles.
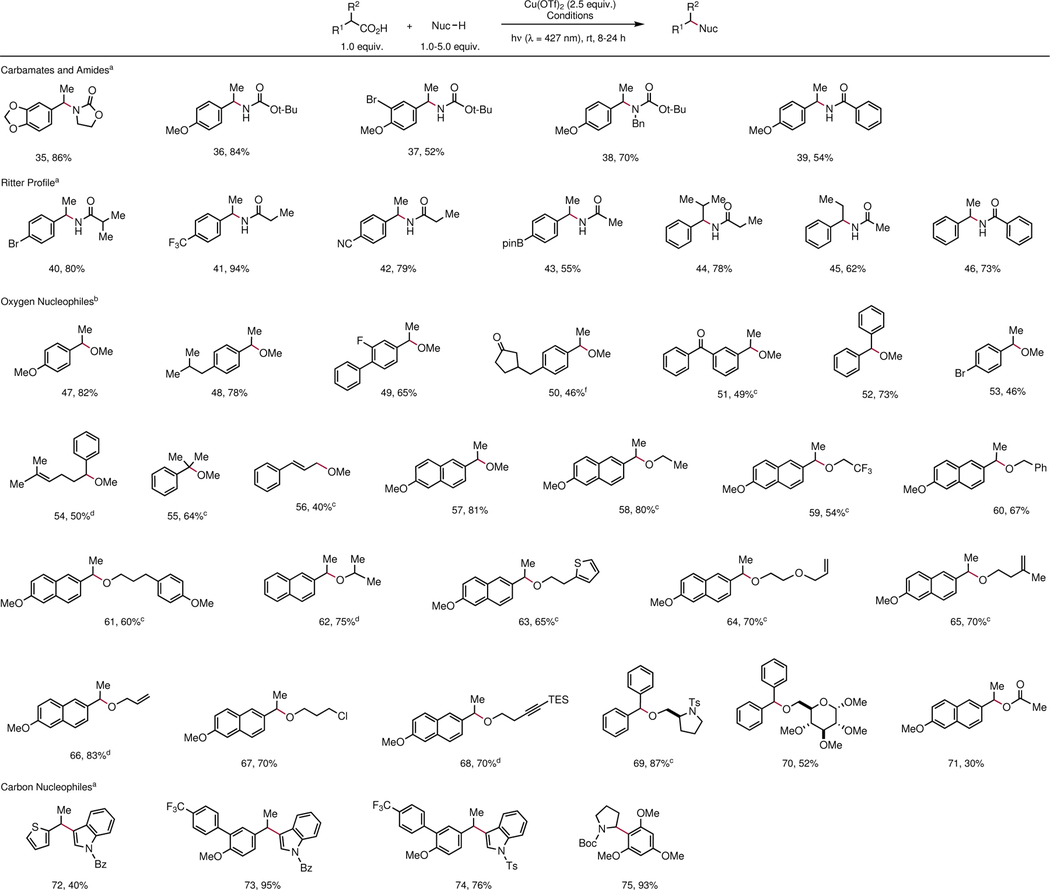
|
Conditions: Cu(OTf)2 (2.5 equiv.), Na3PO4 (3.0 equiv.), nucleophile (1.5 equiv.), carboxylic acid (1.0 equiv.), and MeCN (0.20 M), and are added to a 1.5-dram reaction vial equipped with a stir bar in a glovebox. The vial is irradiated by two 34 W blue LEDs at a distance of 10 cm.
Conditions: Cu(OTf)2 (2.5 equiv.), Na2CO3 (1.0 equiv.), carboxylic acid (1.0 equiv.), and MeCN (0.10 M), and are added to a 1.5-dram reaction vial equipped with a stir bar in a glovebox. The vial is irradiated by two 34 W blue LEDs at a distance of 10 cm.
Diastereomers were not detected in NMR analysis.
Conditions: acid (1.0 equiv.), alcohol (1.0 equiv.), Cu(OTf)2 (2.5 equiv.), and pyridine (3.0 equiv.) are added to Schlenk tubes of 15 cm diameter along with toluene (2.85 mL) and MeCN (150 μL), and the reaction mixture is degassed by freeze-pump-thaw (four 4 min cycles) before irradiation by 34 W blue LED for 8–24 h.
Same conditions in (d), but with 3.0 equiv. alcohol.
Same conditions in (c), but with 5.0 equiv. alcohol. Bpin = pinacolatoboryl.
