Abstract
Derris scandens (DS) is a Thai herbal medicine used to relieve musculoskeletal pain. It has been found as a single crude medication, ethanolic extract, and compounded herbal recipe for oral administration in pharmacies across the country. Due to its medicinal benefits and enriched phytochemicals, researchers are now drawn to examine the new pharmacological effects of this plant to increase its usage in complementary medicines. The purpose of this research was to investigate the wound-healing properties of the plant's ethanolic extracts as well as their active chemical composition. The extracts (both 50% and absolute ethanol) prepared by Soxhlet extraction were examined for cytotoxicity and wound-healing activity using human skin fibroblast cells, and the active chemical contents in the extracts were analyzed further using the HPLC method. For this study, genistein and lupeol compounds were selected as chemical markers. In the concentration range of 0.0001–1 mg/mL, all extracts had no cytotoxic effects on the examined cells, and 1 mg/mL of both ethanolic extracts was effective for wound closure in a scratch assay. The phytochemicals genistein and lupeol were found to be 0.0332% and 0.0588% (w/w) in the 50% ethanolic extract, respectively, and 0.0309% and 0.3472% (w/w) in the absolute ethanolic extract. The ability of DS extracts containing these compounds on in vitro wound-healing activity was demonstrated in this study.
Keywords: Derris scandens, genistein, lupeol, scratch assay, wound healing
INTRODUCTION
Derris scandens (DS) (Roxb.) Benth., also known as Hog Creeper Vine or Thao-Wan-Priang in Thailand, is a member of the Fabaceae family and can be found all over Asia. DS stems are herbal medicines and have a long history of use for pain relief in Thailand and other Asian nations.[1] Many studies have shown that plant extracts are effective in anti-inflammatory, antimicrobial, antioxidant, and anticancer research.[2,3] The presence of active metabolites such as flavonoids and terpenoids was discovered in phytochemical analyses of DS stems.[2] Among these compounds, the isoflavone genistein was identified and issued in Thai Herbal Pharmacopoeia as a marker for the chemical analysis in DS quality control due to its competent bioactivity in anti-inflammatory tests as demonstrated in previous studies.[4,5] Although genistein was reported to have pharmacological effects that contributed to the plant's traditional use, researchers are also interested in exploring the other chemical constituents isolated from DS stems for their related bioactivities, which could elevate the plant as a value-added herb for health promotion. One of the chemical components in the alcoholic extract of DS stems was recently identified as the pentacyclic triterpenoid lupeol.[2] It demonstrated a high cytotoxic effect against colon adenocarcinoma (SW620) cell lines, with an IC50 of 30.50 μg/mL,[6] and a specific anti-inflammatory effect in the 5-LOX enzyme test, with an IC50 of 69.26 μg/mL.[7] With this in mind, these compounds were chosen as chemical markers in the current study, and chemical analysis was performed using Soxhlet extraction and HPLC to examine the contents of these compounds in the ethanolic extracts of DS stems, which were evaluated for their potential on in vitro cytotoxicity and wound-healing activity toward skin fibroblast cells that had not previously been investigated. The purpose of the study was to explore the wound-healing properties of DS extracts for use in skin treatments, as well as the phytochemical components in the extracts that were responsible for the activity.
MATERIALS AND METHODS
Chemicals and materials
DS stems were acquired from a traditional Thai pharmacy in July 2020. By following a process detailed in a previous publication, the acquired sample was authenticated using a genuine specimen deposited at the Department of Pharmacognosy, College of Pharmacy, Rangsit University, Thailand (RSU 0089).[7] Genistein (≥98% purity) was provided by Sigma-Aldrich, Singapore, and lupeol (99% purity) was supplied by Nanjing Spring and Autumn Biological Engineering Co., Ltd., China. All chemical reagents and disposable HPLC instrument accessories were provided by Merck KGaA, Germany, and S.N.P. Scientific Co., Ltd., Thailand, respectively.
Preparation of extracts
The powdered DS stems were mixed and weighed (30 g) before being placed in a Soxhlet extractor thimble. For 3 h of extraction, 1.0 L of 50% (v/v) ethanolic aqueous solution was used as a solvent. The materials were also weighed (30 g) and extracted with the same equipment using the absolute ethanol. Each solvent extraction was performed in triplicate. The extracts, which included 50% and absolute ethanolic solutions, were dried in a rotary evaporator and stored in a desiccator before being precisely weighed.
Cell culture
The normal human skin fibroblasts (HSFs) were provided by the Manose Health and Beauty Research Center Co., Ltd., Thailand. Cells were grown in a complete culture medium including Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 mg/mL) under normal conditions. The cells were incubated at 37°C in a temperature-controlled, humidity chamber (Shel Lab, model 2123TC, Cornelius, OR 97113, USA). The 53rd and 56th passages of cultured cells were employed.
Cell viability by sulforhodamine B assay
In 96-well plates, HSF cells were seeded at a density of 1 × 105 cells/well and incubated overnight. After that, the cells were treated for 24 h with five different extract concentrations (0.0001–1 mg/mL). Ascorbic acid was used as a positive control and was prepared in the same way as the serial extract solutions. After incubation, the adherent cells were fixed by adding 50 μL of cold 50% trichloroacetic acid and incubated for another 1 h at 4°C. The cells were therefore rinsed five times with distilled water, air-dried, and stained with 50 μL of 0.4% sulforhodamine B (SRB) in 1% glacial acetic acid for 30 min at room temperature (27°C ± 2°C). The unbound SRB was eliminated by rinsing four times with a 1% glacial acetic acid solution. Following air drying, the bound dye was dissolved in 10 mM Tris base and mixed, and the absorbance at 540 nm was measured using a well reader (BioTek Synergy HT, USA). The cells that were not given any treatment served as the negative control. The assay was carried out in triplicate, and the percentage of cell viability was calculated using a published method.[8]
Scratch wound migration assay
In a scratch wound migration assay, the maximum concentration of the examined extracts that passed the SRB assay and were nontoxic to HSF cells was chosen. The assay was carried out in accordance with the standard protocol described in previous reports.[9,10] HSF cells were seeded in 24-well plates at a density of 3 × 105 cells/mL, cultured in fibroblast medium containing 10% FBS, and grown to form a confluent cell monolayer. The media was pipetted out and discarded, and the cells were rinsed with phosphate-buffered saline to eliminate any cellular debris after a small area was scraped with a 200 μL pipette tip. The plates were incubated with fibroblast media containing the tested concentration of ethanolic extracts produced from DS stems at 37°C and 5% CO2. Two negative controls, consisting of 10% DMSO and DMEM medium, and one positive control group, consisting of ascorbic acid at a concentration of 1 mg/mL in media, were employed. At 0, 6, 24, and 48 h, the distance between adjacent layers of cells scraped by the pipette tip was measured microscopically. While the HSF cells migrated to cover the scratched region, images were acquired using a digital camera connected to a microscope and computer system. The collected data were evaluated with Motic Images Plus 2.0 software (Motic Asia, Hong Kong).
Preparation of sample solutions and chromatographic apparatus
Dried extracts from 50% ethanol and absolute ethanol extractions were precisely weighed and reconstituted with small amounts of methanol, then diluted further with the same solvent, and adjusted to provide a sample solution with a concentration of 100 mg/mL. The chemical markers genistein and lupeol were accurately weighed and diluted in methanol and then prepared for standard solutions at concentrations of 10, 20, 50, 100, 200, and 400 μg/mL. The sample and serial standard solutions were filtered through a 0.45 μm nylon membrane before analysis. Each filtered sample (20 μL) was injected into an HPLC instrument (1260 Infinity Series, Agilent Technologies, USA), which was equipped with a photodiode array detector. The data collected from the HPLC apparatus were recorded and analyzed using the OpenLab ChemStation software (Agilent Technologies, USA).
Quantification of genistein in extracts
The chromatographic signal of genistein in the extracts was separated from the other compounds on a ZORBAX Eclipse Plus C18 column (150 mm × 4.6 mm i.d., 5 μm) by isocratic mode of the mobile phase system, which composed of methanol and 0.1% (v/v) formic acid in a 52:48 ratio. The HPLC column chamber was held at room temperature, and the mobile phase flow rate was adjusted to 1.0 mL/min. At a wavelength of 260 nm, a signal of the compound was collected. The total analytical time was fixed at 15 min in each sample. The genistein levels estimated from a calibration curve of standard solutions were expressed as a percentage (w/w) of the dried extract in each sample, which was done in triplicate. The analytical method was modified from a previous report, and its accuracy, precision, and specificity were validated using a standard-spiked technique.[11] The samples t-test (PSPP, GNU project) was used to detect genistein differences between sample groups, with a statistical significance of P < 0.05 found among the groups tested.
Quantification of lupeol in extracts
The amount of lupeol found in the extracts was determined using chromatography on the Accucore™ XL C18 column (250 mm × 4.6 mm i.d., 4 μm). The analysis was carried out using an isocratic elution of the mobile phase with a flow rate of 1.0 mL/min of 90:10 methanol and acetonitrile, and a DAD at 210 nm wavelength, as described in our earlier research.[7] The protocol used in the quantitative genistein study was applied to examine lupeol contents, as well as to validate the analytical method and investigate the statistical differences.
RESULTS AND DISCUSSION
Cell viability analysis
After 24 h of treatment, the examined samples from DS stems, including 50% and absolute ethanolic extracts, showed no toxicity in HSF cells. As shown in Table 1, all of the extracts tested, as well as a positive control ascorbic acid, at concentrations of 0.0001, 0.001, 0.01, 0.1, and 1 mg/mL, exhibited satisfactory fibroblast cell growth, with a high percentage of cell viability in the range of 87.30%–115.74%. This indicated that the extracts were nontoxic to HSF cells and that the effect on HSF cell migration in a scratch wound assay could be anticipated. Our findings support the safety of DS extracts for use in human normal cells, as previously reported.[3]
Table 1.
The effect of various Derris scandens extracts and ascorbic acid concentrations on the viability of human skin fibroblast cells
| Extract (mg/mL) | Percent cell viability | ||||
|---|---|---|---|---|---|
|
| |||||
| 0.0001 | 0.001 | 0.01 | 0.1 | 1 | |
| 50% ethanol | 103.70±0.85 | 101.88±1.57 | 99.08±1.07 | 94.45±3.03 | 89.41±2.47 |
| Absolute ethanol | 91.81±2.11 | 102.32±1.56 | 108.41±4.46 | 110.17±2.45 | 115.74±3.39 |
| Ascorbic acid | 110.69±2.84 | 109.03±2.18 | 107.62±0.77 | 106.67±2.69 | 87.30±1.19 |
Mean±SD, n=3. SD: Standard deviation
Wound-healing properties evaluated via scratch wound assay
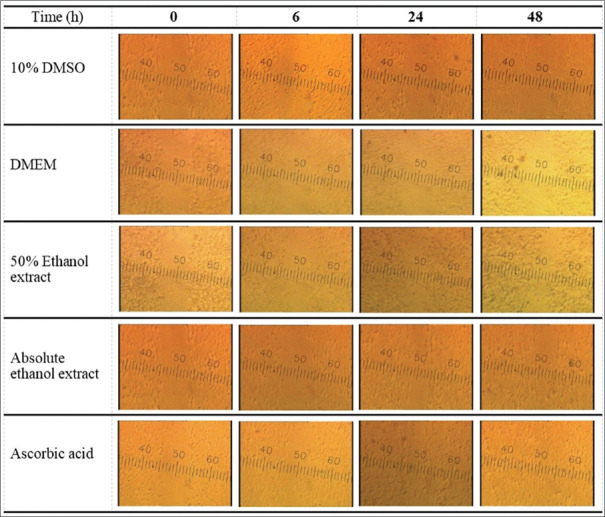
The migratory property of HSF cells treated with DS extracts was investigated using a scratch wound assay. This experiment used the highest concentration of DS extracts (1 mg/mL) that showed no cytotoxic effect in the SRB assay. The potential wound-healing effects of two different extracts (50% and absolute ethanol) and the controls including ascorbic acid, 10% DMSO, and DMEM were evaluated within 48 h. After the scratch, the migration of HSF cells to envelop the simulated wound was clearly visible at 24 h in both 50% and absolute ethanolic samples, whereas ascorbic acid, a positive control in this study, induced cell migration almost completely at the same time of observation. Both ethanolic extracts performed almost entire cell migration in the 48 h of observation. As shown in Figure 1, cell migration was more evident in all tested extracts and the positive control treatment group than in other controls. Our findings revealed that two different DS extracts at 1 mg/mL had wound-healing effects on HSF cells, but that the time required to conduct wound closure was longer for the examined extracts than for the positive control used in this study. The efficacy of ascorbic acid as a fibroblast migration enhancer was demonstrated, consistent with prior studies.[12]
Figure 1.
With a wound scratch assay, a series of photographs depicting the influence of various tested samples on human skin fibroblasts cell migration
Quantification of bioactive compounds in Derris scandens extracts
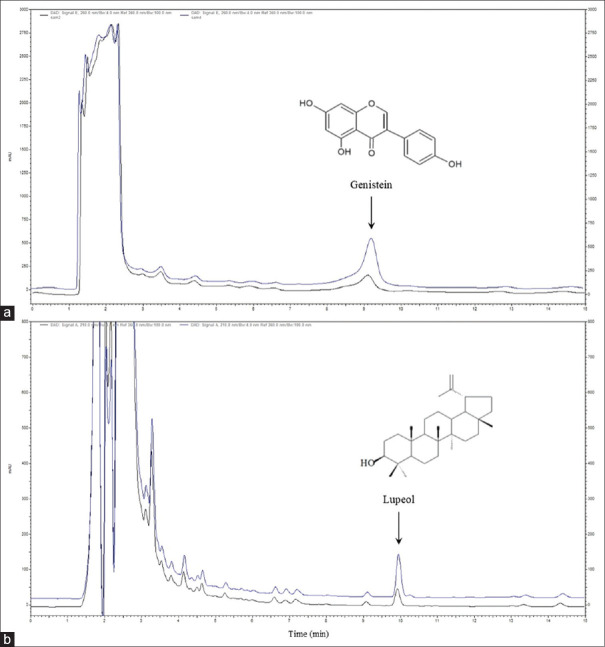
In both 50% and absolute ethanolic extracts, HPLC chromatograms displayed peaks of the selected compounds genistein and lupeol at retention times of 9.2 and 9.9 min, respectively. Due to their chemical structure and polarity, the appropriate conditions for studying these compounds were determined individually. The analytical procedures employed in this study were accurate and precise, providing acceptable recovery and relative standard deviation percentages of average values of 102.16% and 1.04% for genistein and 95.20 and 0.92% for lupeol, respectively. Method specificity was also proved by employing a standard-spiked approach, as shown in Figure 2. As summarized in Table 2, 50% ethanol yielded percentages of genistein and lupeol per dried extract (%w/w) of 0.0332 ± 0.0014 and 0.0588 ± 0.0014, respectively. The yields of genistein and lupeol in absolute ethanol were 0.0309% ± 0.0009% and 0.3472% ± 0.0036%, respectively. While the contents of genistein and lupeol in both ethanolic samples differed considerably, the difference in genistein content found among the extracts was not significant (P < 0.05). Lupeol yield was detected in all extracts to a much greater extent than genistein, implying that the lupeol compound is evident as an important constituent in DS extracts in the same manner that genistein, a primitive chemical marker, has been present.[3,4,5] As a result, lupeol could be an efficient alternative marker for DS extract quality control. Moreover, when the percentage of ethanol in the extraction was raised, the ratio of lupeol to genistein increased approximately six-fold. The findings suggest that the polarity of the solvents employed in extraction has an impact on the yield of active compounds with diverse chemical structures. For the extraction of the triterpene lupeol, a high percentage of ethanol is favored; however, for the extraction of the isoflavone genistein, which has greater polarity than lupeol, a substantial amount of ethanol is unaffected. These compounds are supposed to facilitate wound healing because their actions have been attributed to their ability to stimulate cell migration, which includes processes such as reduced oxidative stress and modulated proinflammatory cytokines.[13,14] As a consequence, identifying these compounds could probably determine that they were responsible for the activity. The difference in constituent ratios had no effect on wound-healing ability in either of the ethanolic extracts, which also produced positive results in this study.
Figure 2.
Overlay chromatograms showing the peak of chemical markers genistein (a) and lupeol (b) from Derris scandens extracts: A standard-spiked sample (upper line) and a blank sample (bottom line)
Table 2.
The content of chemical markers in different Derris scandens extracts
| Extract | Contents (%w/w) | ||
|---|---|---|---|
|
| |||
| Genistein (G) | Lupeol (L) | L/G ratio | |
| 50% ethanol | 0.0332±0.0014 | 0.0588±0.0014 | 1.77 |
| Absolute ethanol | 0.0309±0.0009 | 0.3472±0.0036 | 11.24 |
Mean±SD, n=3. SD: Standard deviation
CONCLUSION
The presence of the isoflavone genistein and the triterpene lupeol in ethanolic extracts of DS stems, which have nontoxic and wound-healing properties in HSF cells, is the first demonstration. Extensive pharmacological research on the extracts would be useful in providing a rationale for their future development as herbal skin-repair treatments.
Financial support and sponsorship
This research was supported by the Research Institute of Rangsit University, Thailand.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
For providing the necessary resources, authors thank the Rangsit University Research Institute.
REFERENCES
- 1.Kuptniratsaikul V, Pinthong T, Bunjob M, Thanakhumtorn S, Chinswangwatanakul P, Thamlikitkul V. Efficacy and safety of Derris scandens Benth extracts in patients with knee osteoarthritis. J Altern Complement Med. 2011;17:147–53. doi: 10.1089/acm.2010.0213. [DOI] [PubMed] [Google Scholar]
- 2.Hussain H, Al-Harrasi A, Krohn K, Kouam SF, Abbas G, Shah A, et al. Phytochemical investigation and antimicrobial activity of Derris scandens. J King Saud Univ Sci. 2015;27:375–8. [Google Scholar]
- 3.Nooin R, Pitchakarn P, Kanchai C, Jaikang C. Assessments of antioxidant, antilipid peroxidation, and in-vitro safety of Derris scandens vine extracts from Southern Thailand. Pharmacogn Res. 2019;11:60–6. [Google Scholar]
- 4.Traipop S, Chuanuwatanakul S, Chailapakul O, Punrat E. Facile and fast detection of genistein in Derris scandens by square wave voltammetry using a cobalt (II) phthalocyanine-modified screen-printed electrochemical sensor. Curr Anal Chem. 2020;16:341–8. [Google Scholar]
- 5.Ayameang O, Rattarom R, Mekjaruskul C, Caichompoo W. Anti-inflammatory activity and quantitative analysis of major compounds of the mixtures of Derris scandens (DZSS) formula. Pharmacogn J. 2020;12:828–34. [Google Scholar]
- 6.Somwong P, Chuchote C. Determination of lupeol, a cytotoxic compound against SW620 cells in the extracts of Ha-Rak recipe. Pharmacogn J. 2021;13:133–8. [Google Scholar]
- 7.Somwong P, Theanphong O. Quantitative analysis of triterpene lupeol and anti-inflammatory potential of the extracts of traditional pain-relieving medicinal plants Derris scandens, Albizia procera, and Diospyros rhodocalyx. J Adv Pharm Technol Res. 2021;12:147–51. doi: 10.4103/japtr.JAPTR_13_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaikul P, Khat-Udomkiri N, Iangthanarat K, Manosroi J, Manosroi A. Characteristics and in vitro anti-skin aging activity of gallic acid loaded in cationic CTAB niosome. Eur J Pharm Sci. 2019;131:39–49. doi: 10.1016/j.ejps.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Muhammad AA, Pauzi NA, Arulselvan P, Abas F, Fakurazi S. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. Biomed Res Int. 2013;2013:974580. doi: 10.1155/2013/974580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syarina PN, Karthivashan G, Abas F, Arulselvan P, Fakurazi S. Wound healing potential of Spirulina platensis extracts on human dermal fibroblast cells. EXCLI J. 2015;14:385–93. doi: 10.17179/excli2014-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasparetto JC, Smolarek FS, de Francisco TM, Miranda LC, Pontarolo R, Siqueira PF. Development and validation of an HPLC–DAD method for analysis of the six major isoflavones in extracts from soybean processing. J Am Oil Chem Soc. 2012;89:1211–22. [Google Scholar]
- 12.Mohammed BM, Fisher BJ, Kraskauskas D, Ward S, Wayne JS, Brophy DF, et al. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int Wound J. 2016;13:572–84. doi: 10.1111/iwj.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park E, Lee SM, Jung IK, Lim Y, Kim JH. Effects of genistein on early-stage cutaneous wound healing. Biochem Biophys Res Commun. 2011;410:514–9. doi: 10.1016/j.bbrc.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Pereira Beserra F, Xue M, Maia GL, Leite Rozza A, Helena Pellizzon C, Jackson CJ. Lupeol, a pentacyclic triterpene, promotes migration, wound closure, and contractile effect in vitro: Possible involvement of PI3K/Akt and p38/ERK/MAPK pathways. Molecules. 2018;23:2819. doi: 10.3390/molecules23112819. [DOI] [PMC free article] [PubMed] [Google Scholar]