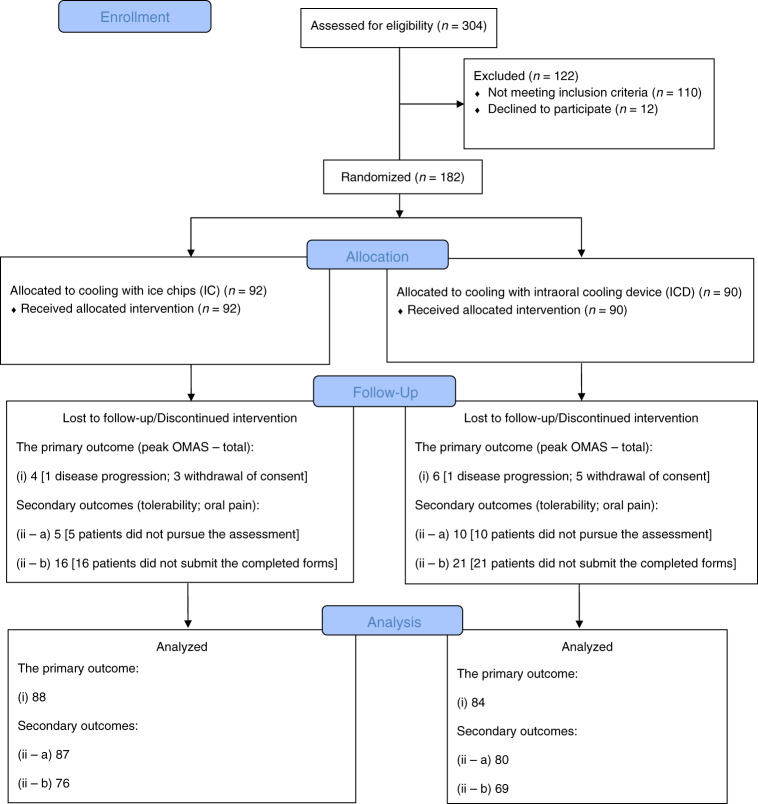
Fig. 1. Flow diagram.
In total, ten patients (n = 10) dropped out of the enrolled study sample (n = 182; IC = 92; ICD = 90) related to the primary outcome (i) [n = 172; IC = 88; ICD = 84]. Two patients (n = 2; 1 in each intervention arm) did not continue the study due to fatal outcome related to disease progression. Eight patients (n = 8; 3 in the IC group and 5 in the ICD group) withdrew their consent to participate in the study. For the secondary outcome tolerability (ii–a) [n = 167; IC = 87; ICD = 80], 15 (n = 15; 5 in the IC group and 10 in the ICD group) patients were not able to pursue the assessment; for the secondary outcome patient-reported oral pain (ii–b) [n = 145; IC = 76; ICD = 69], 37 (n = 37; 16 in the IC group and 21 in the ICD group) did not submit the completed forms. IC, ice chips; ICD, intraoral cooling device.