Figure 2.
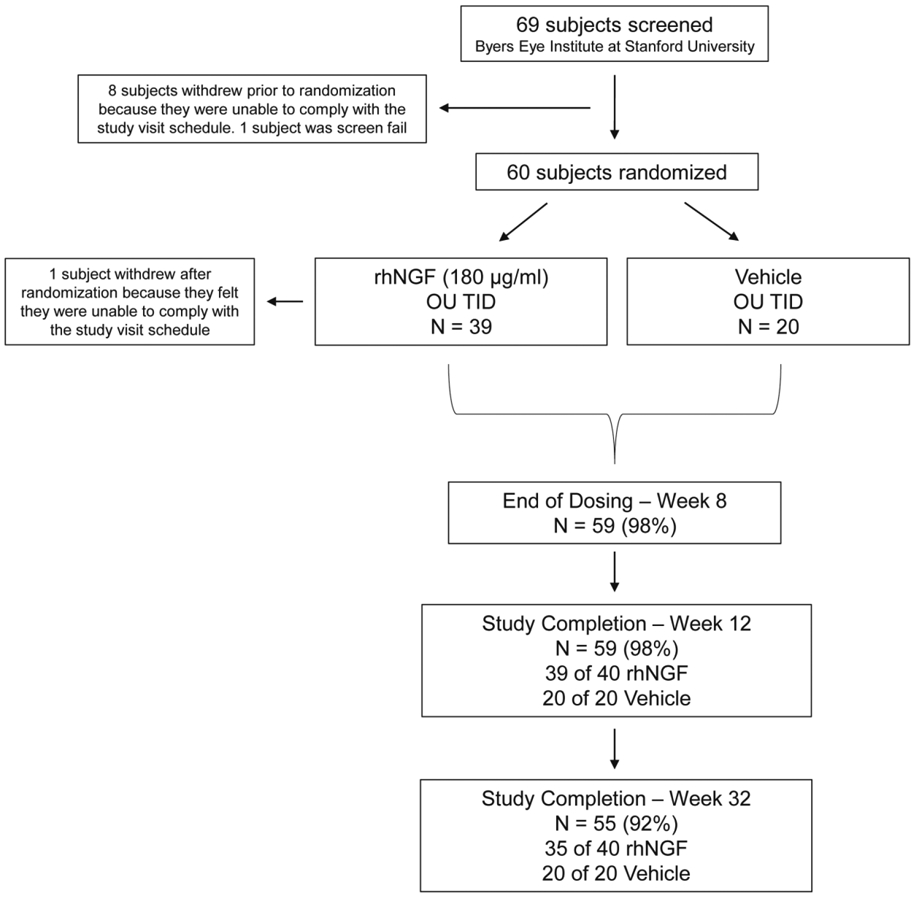
Study design and patient disposition. In sixty (60) open-angle glaucoma patients, one eye was officially selected as the study eye although both eyes underwent dosing and data collection. Primary endpoints were safety, as assessed through adverse events, and tolerability, as assessed through Visual Analogue Scale (VAS). Secondary objectives were to measure changes in best corrected visual acuity (BCVA), Humphrey visual field (HVF), electroretinography (ERG), and optical coherence tomography (OCT) at baseline and at the week 8, 12, and 32 visits. Exploratory objectives included flavoprotein fluorescence and adaptive optics imaging and OCT angio. Results through week 32 for all subjects are reported.
