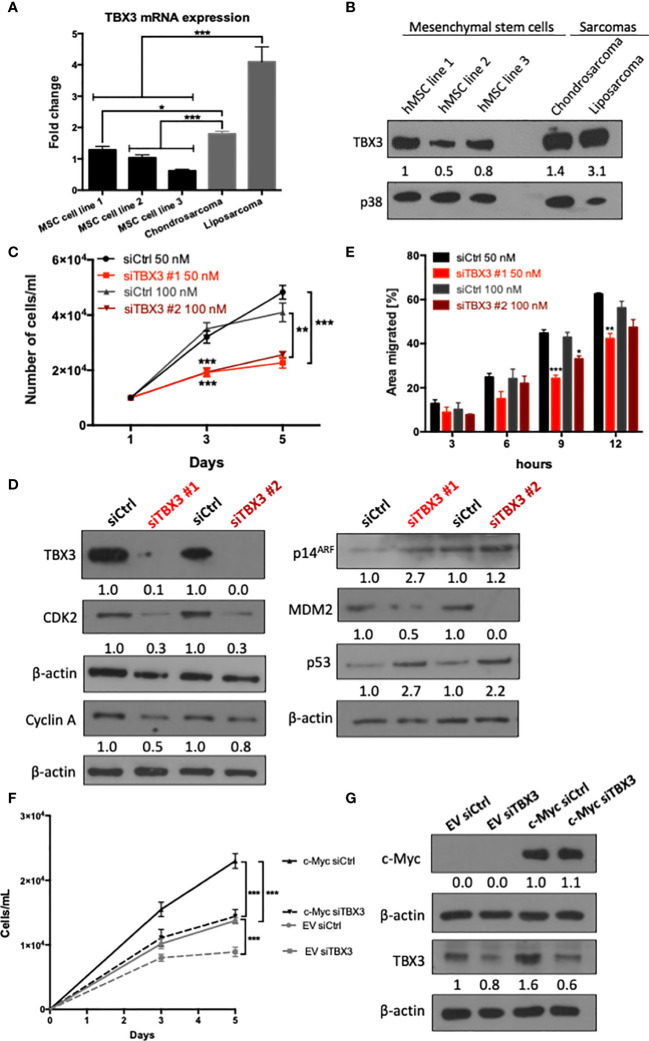
Figure 2.
TBX3 contributes to hMSC proliferation and migration. (A) qRT-PCR analysis of TBX3 mRNA expression in adipose-derived hMSCs from three different donors (MSC cell lines 1-3) as well as chondrosarcoma (SW1353) and liposarcoma (SW872) cells. (B) Western blot analysis of TBX3 protein expression in the cells described in (A). p38 was used as a loading control. (C–E) hMSCs were transiently transfected with siTBX3 or siControl (siCtrl) and (C) Growth curve assays were performed over a 5-day period; (D) Western blotting of protein harvested from cells in (C) on day 5 with antibodies to TBX3, CDK2, cyclin A, p14ARF, MDM2, and p53; and (E) 2D-Scratch motility assays were performed where a linear wound was made on confluent transiently transfected hMSCs and distance migrated was measured at 3, 6, 9 and 12 h. (F, G) hMSCs were stably transduced with EV or a c-Myc lentiviral expression construct and transiently transfected with siTBX3 or siControl (siCtrl). (F) Growth curve assays were performed over a 5-day period; (G) Western blotting of protein harvested from cells in (F) on day 5 with antibodies to c-Myc and TBX3. For western blotting, β-actin was used as a loading control and densitometry readings were obtained using Fiji and protein expression levels are represented as a ratio of protein of interest/p38 or protein of interest/β-actin normalized to hMSC line 1 or siCtrl respectively. Student’s t-test was used to compare between groups, *p < 0.05; **p < 0.01; ***p < 0.001; error bars represent mean ± SEM (n=3, for all panels).