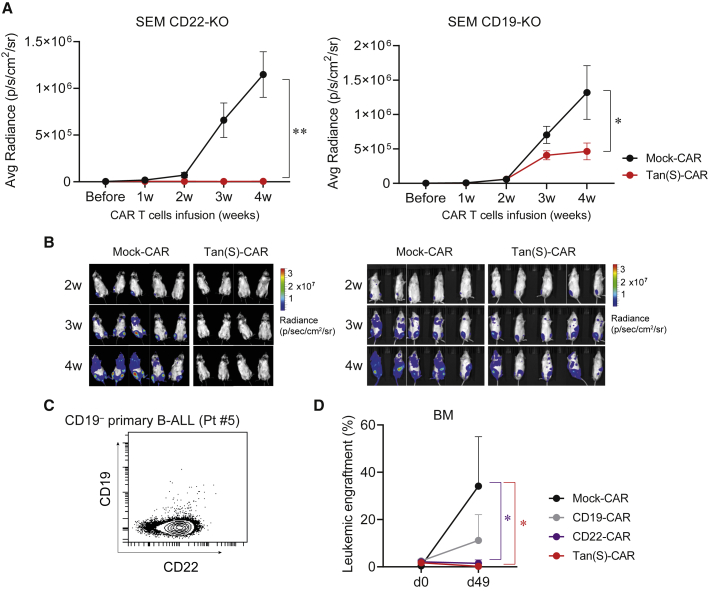
Figure 6.
In vivo bispecificity of Tan(S)-CAR T cells
(A) Average radiance quantification (p/sec/cm2/sr) from mice transplanted with SEM CD22-KO (n = 4–5/group) or SEM CD19-KO (n = 9–10/group) at the indicated time points after Mock- or Tan(S)-CAR T cell infusion. Data from n = 2 independent experiments. (B) Representative BLIs of SEM CD22-KO- and SEM CD19-KO-transplanted mice from week 2 to week 4 after CAR T cell infusion. (C) CD19 and CD22 expression of the CD19−CD22+ blasts from the B-ALL patient (Pt#5) used for the in vivo bispecificity experiment. (D) NSG mice (n = 6/group) were i.v. transplanted with 1 × 106 of CD19−CD22+ B-ALL cells (Pt#5). Upon detectable B-ALL engraftment in BM, mice were randomized to receive 5 × 106 Mock-CAR, CD19-CAR, CD22-CAR, or Tan(S)-CAR T cells. The leukemic burden in BM before CAR T cell infusion (day 0) and at endpoint of the experiment (day 49) is shown. Data are shown as means ± SEMs. ∗p < 0.05, ∗∗p < 0.01, 2-way ANOVA (mixed-effects model) with Šídáks multiple comparisons test.