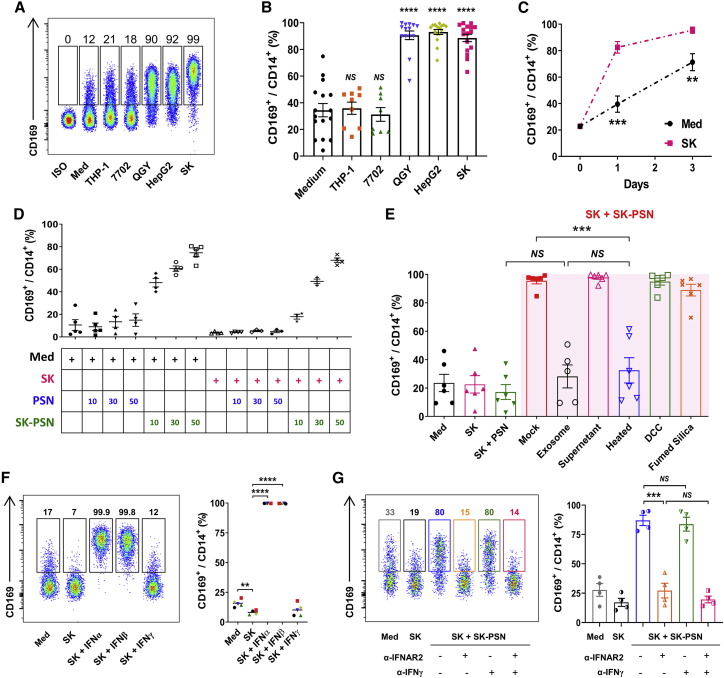
Figure 4.
Type I IFN could induce CD169 upregulation on tumor-exposed macrophages
(A and B) PBMCs purified by the adherent treatment method were cultured with the indicated TCS for 2 days. Flow cytometry patterns and gate frequencies in percentages (A) and statistical results (B) are shown; n = 16. (C) PBMCs enriched by using the adherent treatment method were cultured with medium or SK TCS for 0–3 days, after which the ratio of CD169+ cells in CD14+ cells was determined by flow cytometry; n = 6 or 7. (D) Supernatants from PBMCs pretreated with medium or SK TCS for 48 h were collected (designated as PSN and SK-PSN, respectively). Thereafter, CD14+ monocytes were isolated with magnetic bead sorting and cultured with medium, SK TCS, PSN, or SK-PSN for 48 h; n = 4 or 5. (E) CD14+ monocytes were exposure to different fluid components for 2 days, after which the CD169+ cell ratio was determined by flow cytometry; n = 6. The exosome and supernatant components of SK-PSN were separated by ultracentrifugation; the proteins, lipids, and small molecules in SK-PSN were removed by high-temperature treatment (100°C, 30 min), fumed silica, and DCC, respectively. (F) CD14+ monocytes were cultured with medium, SK TCS, or 10 pg/mL IFNα2/IFNβ/IFNγ, respectively. Two days later, the ratio of CD169+ cells in CD14+ cells was determined by flow cytometry; n = 5. (G) CD14+ monocytes were cultured in the presence or absence of 5 μg/mL anti-IFNAR2 or 1 μg/mL anti-IFNγ in various conditions. Two days later, the ratio of CD169+ cells in CD14+ cells was determined by flow cytometry; n = 4. Data represent mean ± SEM. ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; NS, not significant by multiple t test (C) and an unpaired Student’s t test (B and E–G).