Abstract
Background:
Cell-based cartilage restoration with autologous chondrocyte implantation (ACI) is a safe and effective treatment for symptomatic cartilage lesions. Many patients undergoing ACI have a history of prior surgery, including bone marrow stimulation (BMS). There is mounting evidence that a history of prior BMS may impede healing of the ACI graft.
Purpose/Hypothesis:
The purpose of this study was to compare the failure rates of primary ACI with ACI after prior BMS. We hypothesized that ACI after BMS would have a significantly higher failure rate (defined as reoperation, conversion to arthroplasty, and/or imaging-based failure) compared with primary ACI.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A literature search was performed by use of PubMed and Embase databases for relevant articles published through October 2, 2020, to identify studies evaluating outcomes and failures rates of ACI after prior BMS in the knee.
Results:
Included were 11 studies comprising 1479 ACI procedures. The mean age at surgery ranged from 18.3 to 39.1 years, and the mean follow-up ranged from 3 to 20.6 years. All studies reported failure rates. The overall failure rate was significantly higher in the patients who underwent ACI after BMS, at 26.4% compared with 14.8% in the ACI group (P < .001). Meta-analysis demonstrated an increased risk of failure in patients with a history of prior BMS (log odds ratio = –0.90 [95% confidence interval, –1.38 to –0.42]).
Conclusion:
This systematic review demonstrated that failure rates were significantly higher for patients treated with ACI after BMS relative to patients undergoing ACI without prior BMS. This finding has important implications when considering the use of BMS for defects that are amenable to cell-based restoration and when determining treatment options after failed BMS.
Registration:
PROSPERO (CRD42020180387).
Keywords: articular cartilage, autologous chondrocyte implantation, microfracture
Cartilage lesions in the knee are a common problem, present in up to 75% of knees at the time of arthroscopy. 36,40 One analysis of >30,000 knee arthroscopies showed there is an incidence of 41% grade 3 and 19% grade 4 changes, 6 demonstrating that many of these lesions are advanced and have the potential for significant morbidity. Furthermore, cartilage lesions are difficult to treat because of the poor intrinsic healing potential of chondrocytes, and untreated cartilage lesions are known to cause morbidity related to pain, swelling, mechanical obstruction, and ultimate development of osteoarthritis. 7,33 Surgical treatment options for symptomatic cartilage lesions range from surface treatments, such as arthroscopic debridement, to bone marrow stimulation (BMS) techniques, such as microfracture, and restorative techniques, such as autologous chondrocyte implantation (ACI), osteochondral autograft transfer system (OATS), and osteochondral allograft.
Marrow stimulation procedures, such as microfracture, were popularized by Steadman and colleagues 37 and have long been considered the gold standard for initial management of most grade 3 to 4 cartilage lesions. BMS using methods such as microfracture exposes subchondral bone marrow and its mesenchymal stem cells to induce healing of a fibrocartilaginous layer over the cartilage lesion. 38 Traditionally, this has been an effective treatment for smaller-sized lesions in the short-term follow-up, and it is often viewed as a low-morbidity surgery. 12,39 The durability of BMS is questioned because outcomes do seem to deteriorate in medium-term follow-up, which may result in patients needing revision surgery. 3,10,12
Restorative techniques, such as ACI or osteochondral transfer, have typically been seen as second-line treatments because of their increased surgical morbidity, rigorous recovery, and resource intensity. These options are often considered for patients with ongoing symptoms after BMS. ACI relies on implantation of a cartilaginous matrix onto intact subchondral bone, and some have speculated that an induced fibrocartilaginous scar from prior microfracture may inhibit healing of ACI graft and thus lead to inferior outcomes. 15,20,32,33 Despite this idea, there have been variable reports of clinical outcomes regarding ACI following prior BMS.
The purpose of this study was to systematically review the literature on patients undergoing ACI after a previous BMS procedure to identify the influence of prior BMS on eventual ACI failure. We hypothesized that reported failure rates would be significantly higher for patients with a history of prior BMS relative to patients with ACI without prior BMS.
Methods
Study Identification
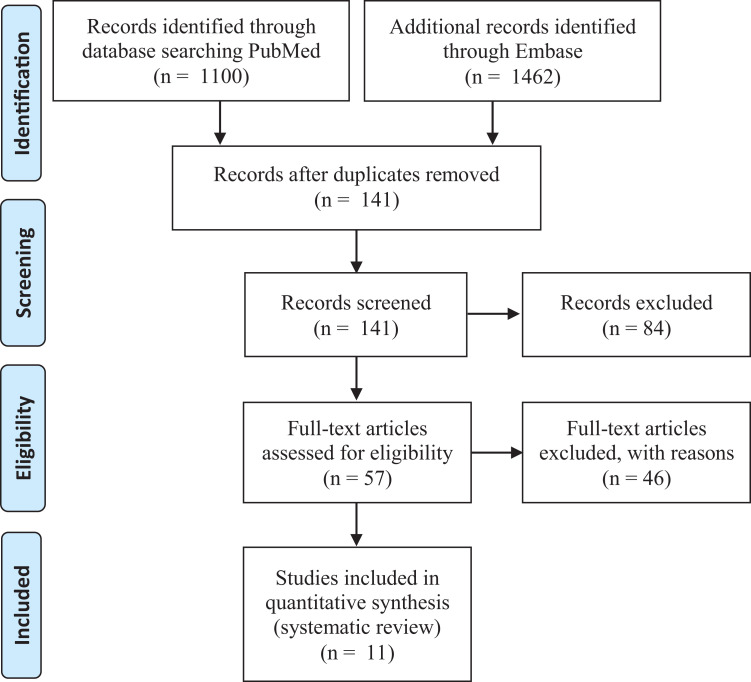
A systematic review was performed in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. To identify all relevant articles, we used the following search terms: (“revision” or “failed”) AND (“cartilage” or “chondral” or “osteochondral” or “ACI” or “MACI” or “autologous chondrocyte”) and (“knee”). This literature search was performed by 2 independent authors (C.J.C. and J.F.), and the PubMed and Embase databases were searched through October 2, 2020. Article titles were first reviewed independently by the same 2 authors to determine potential inclusion. The abstract for any article selected by either reviewer was then reviewed for appropriateness. Finally, the full manuscript for any study with an abstract selected by either reviewer was reviewed independently by the same 2 authors for inclusion. There was also a manual search through references of the selected studies for any additional studies that may have been missed with the database search. Figure 1 shows the study selection process. This study was registered on PROSPERO (CRD42020180387).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Studies were included if they were primary research articles written in the English language; evaluated patients undergoing knee ACI after prior BMS; and reported validated outcome measures, including report of failure. Duplicate manuscripts were excluded; however, some authors published data on aging cohorts at different time points, and these studies were included as separate cohorts. Of note, studies that included ACI with a concomitant procedure were included. Studies were excluded if they were not primary research articles, they did not include a subgroup analysis for patients undergoing ACI with prior BMS, or the full text could not be acquired.
Data Extraction
A standardized data extraction form was compiled independently on a prespecified data form by 2 authors (C.J.C. and J.F.). The data collected for analysis included year of publication, level of evidence, basic demographics (age, sex, body mass index), lesion location, lesion size, number of lesions, procedures, generation of ACI technique, follow-up time, concomitant procedures, clinical outcomes, and survivorship. A quality assessment of each study was performed using the MINORS (methodological index for non-randomized studies) criteria. 35 All discrepancies about data were resolved by consensus after the initial data collection was performed.
Statistical Analysis
Failure rates were extracted when described for patients with a history of prior BMS and for those undergoing primary ACI. A meta-analysis and funnel plot were performed using a random-effects model. The relative likelihood of failure was estimated using the log odds ratio, including the 95% confidence interval. Data heterogeneity was determined using I 2, and significance was defined as P < .05. Statistical analysis was performed using Stata Version 16.1 (StataCorp).
Results
Study Characteristics
A total of 11 studies were included in this review (Tables 1 and 2). The level of evidence varied among these studies, with 2 level 2 studies, 20,41 4 level 3 studies, 15,23,32,33 and 5 level 4 studies. 4,21,26,29,31 Publication dates ranged between 2009 and 2020. The mean age of patient cohorts in each study ranged from 18.3 to 39.1 years. Mean follow-up ranged from 3 to 20.6 years. Minimum follow-up was 1.3 years, and maximum was 21 years. One study included only unipolar femoral condyle lesions, 4 2 studies included only patellofemoral lesions, 26,31 3 studies excluded patients with lesions of the patella, 4,9,41 and 6 studies had no exclusion criteria based on location of injury within the knee. 15,20,21,23,32,33 Mean lesion size ranged from 4.2 to 11.8 cm2. For 7 of the studies 15,20,23,31 -33,41 included in this review, mean lesion size ranged from 4.2 to 5.6 cm2. Four studies 4,21,26,29 reported a mean lesion size >8 cm2.
Table 1.
Included Studies a
| Lead Author | Journal | Year | Country | Study Design | Level of Evidence | MINORS Score |
|---|---|---|---|---|---|---|
| Minas 20 | AJSM | 2009 | USA | Prospective cohort | 2 | 19 |
| Zaslav 41 | AJSM | 2009 | USA | Prospective cohort | 2 | 9 |
| Riff 33 | AJSM | 2020 | USA | Retrospective cohort | 3 | 16 |
| Müller 23 | KSSTA | 2020 | Germany | Prospective cohort | 3 | 17 |
| Pestka 32 | AJSM | 2012 | Germany | Retrospective cohort | 3 | 15 |
| Jungmann 15 | AJSM | 2012 | Germany | Retrospective cohort | 3 | 5 |
| Ogura 26 | AJSM | 2019 | USA | Case series | 4 | 15 |
| Beck 4 | Adv Orthop | 2018 | USA | Retrospective cohort | 4 | 6 |
| Ogura 29 | AJSM | 2017 | USA | Case series | 4 | 9 |
| Minas 21 | CORR | 2014 | USA | Prospective cohort | 4 | 10 |
| Pascual-Garrido 31 | AJSM | 2009 | USA | Case series | 4 | 18 |
a Adv Orthop, Advances in Orthopedics; AJSM, American Journal of Sports Medicine; CORR, Clinical Orthopaedics and Related Research; KSSTA, Knee Surgery, Sports Traumatology, Arthroscopy; MINORS, methodological index for non-randomized studies.
Table 2.
Study Characteristics a
| Lead Author | Age, y Mean ± SD (range) |
Site of Lesion, % | Follow-up, y Mean ± SD (range) |
N | Sex (M/F), n | Prior Cartilage Procedures | Defect Size, cm2, Mean ± SD (range) |
Workers’ Compensation, n (%) |
|---|---|---|---|---|---|---|---|---|
| Minas 20 | 35 ± 9.2 (13-60) a 35.4 ± 10.1 (14-55) b | – | 4.5 ± 2.3 (2-11)
a
4.7 ± 2.5 (2-12) b |
325 | 185/136 | MFX (n = 25) AA (n = 33) Drill (n = 53) |
4.6 ± 2.7 (0.5-21)
a
5.2 ± 3.1 (0.7-16.8) b |
28 (13)
a
24 (22) b |
| Zaslav 41 | 35.5 ± 8.6
a
32.9 ± 7.6 b |
MFC (64-68) LFC (16-18) Troch (16-18) |
3.8 ± 2.4 | 143 | 106/48 | MFX (n = 42) Drill (n = 16) Other (n = 2) AA (n = 9) |
4.7 ± 2.1
a
4.6 ± 4.1 b |
– |
| Riff 33 | 30.4 ± 9.4
a
30.3 ± 9 (14.9-49.9) b |
MFC (29-38) LFC (10-18) Troch (25-28) Pat (19-25) |
3.6 ± 1.7
a
3.9 ± 2.0 b |
192 | 102/90 | BMS | 5.0
a
5.0 b |
24 (25)
a
23 (25) b |
| Müller 23 | 32.9 ± 11.8 (16-55)
a
39.1 ± 10 (19-53) b |
Femoral (50-55) Pat (40-45) Troch (5) |
3 | 40 | 14/26 | BMS | 5.4 ± 2.6 (2-15)
a
4.8 ± 2 (2-10) b |
– |
| Pestka 32 | 33.6 ± 10.1 (19.2-54.2)
a
34.1 ± 9 (14.8-45.8) b |
MFC (57.1) LFC (7.1) Troch (2) Pat (10.7) |
3.5 ± 1.4 (1.3-7)
a
4 ± 1.4 (1.3-6.3) b |
55 | 32/24 | MFX (n = 28) | 4.7 ± 1.6 (2.5-9.0)
a
4.6 ± 2.7 (1.5-7.5) b |
– |
| Jungmann 15 | 34.9 ± 9.0 | – | 4.4 ± 0.9 (2-11.8) | 383 | 237/176 | BMS | 5.6 ± 3.0 | – |
| Ogura 26 | 36.6 ± 9.2 (16-55) | Bipolar PF (100) | 8.8 ± 4.2 (2-16) | 60 | 34/22 | BMS | 10.2 ± 4.1 (2.7-19.7) | – |
| Beck 4 | 18.3 ± 0.2 (15-22) | MFC (60) LFC (40) |
12 ± 4.5 | 10 | 5/5 | BMS | 9.1 ± 2.0 (2.3-24) | – |
| Ogura 29 | 35.4 ± 10.4 (13-52) | – | 20.6 ± 0.3 (20-21) | 24 | 16/7 | BMS | 11.8 ± 8 (2.4-30.5) | 8 (33) |
| Minas 21 | 35.8 ± 9.6 (8-57) | – | 12 ± 2 | 210 | 113/97 | MFX (n = 13) AA (n = 30) Drill (n = 46) |
8.4 ± 5.5 | 46 (22) |
| Pascual-Garrido 31 | 31.8 ± 8.6 | – | 4 (2-7) | 37 | 26/26 | MFX | 4.2 ± 1.6 | – |
a Control group. Dashes indicate data was not available. AA, abrasion arthroplasty; BMS, bone marrow stimulation; LFC, lateral femoral condyle; M/F, male/female; MFC, medial femoral condyle; MFX, microfracture; Pat, patella; PF, patellofemoral; Troch, trochlea.
b BMS group.
Failure Rates
Failure was defined in a variety of ways, including reoperation, conversion to arthroplasty, imaging characteristics, patient-reported outcomes (PROs), or clinical symptoms (Table 3). For definitions of failure, 7 studies 20,21,26,29,31,33,41 included conversion to arthroplasty, 5 studies 15,20,26,29,31 included imaging-based criteria, 10 studies 4,20,21,23,26,29,31 –33,41 included reoperation, 1 study 15 included clinical assessment, and 1 study 41 included a PRO-based definition of failure.
Table 3.
Failure and Outcome Data a
| Lead Author | Definition of Failure | Outcome Measure |
|---|---|---|
| Minas 20 | Conversion to arthroplasty, imaging based, reoperation | Treatment failure |
| Zaslav 41 | Conversion to arthroplasty, PRO based, reoperation | mCKR, KOOS |
| Riff 33 | Conversion to arthroplasty, reoperation | Tegner, Lysholm, IKDC, KOOS, SF-12 |
| Müller 23 | Reoperation | IKDC, VAS |
| Pestka 32 | Reoperation | IKDC, KOOS, VAS |
| Jungmann 15 | Clinical assessment, imaging based | Treatment failure |
| Ogura 26 | Conversion to arthroplasty, imaging based, reoperation | mCKR, WOMAC, VAS, SF-36 |
| Beck 4 | Reoperation | IKDC, KOOS, mCKR |
| Ogura 29 | Conversion to arthroplasty, imaging based, reoperation | mCKR, WOMAC, SF-36 |
| Minas 21 | Conversion to arthroplasty, reoperation | mCKR, WOMAC, KSS, SF-36 |
| Pascual-Garrido 31 | Conversion to arthroplasty, imaging based, reoperation | Lysholm, IKDC, KOOS, SF-12, mCKR, Tegner |
a IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; KSS, Knee Society Score; mCKR, modified Cincinnati Knee Rating; PRO, patient-reported outcome; SF-12, 12-Item Short Form Health Survey; SF-36, 36-Item Short Form Health Survey; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
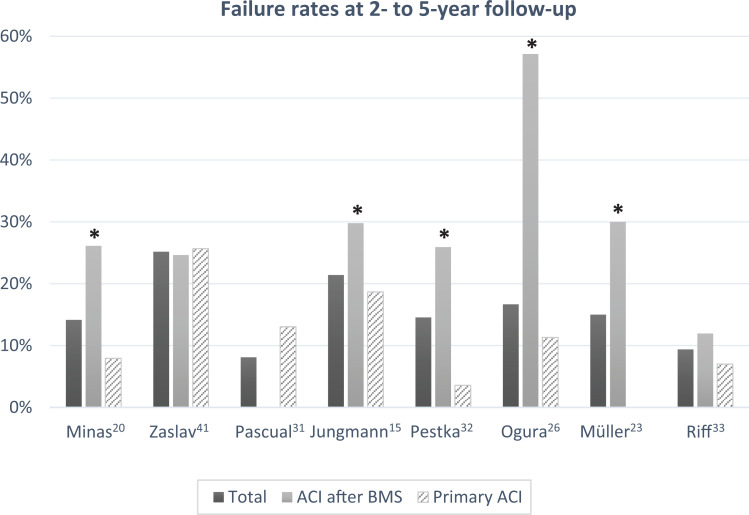
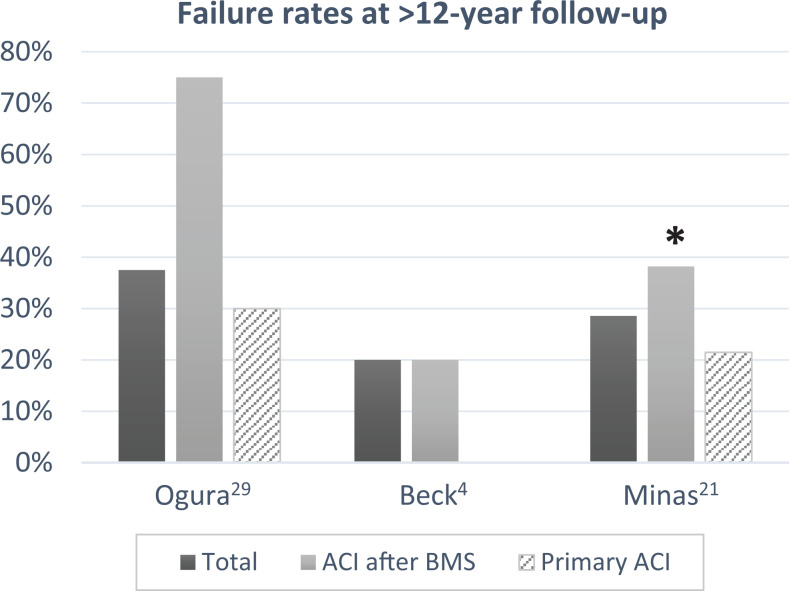
Failure rates were higher for patients undergoing a prior microfracture in all except 2 studies. 31,41 This included studies within the range of 2- to 5-year follow-up (Figure 2) as well as those with >12-year follow-up data (Figure 3). For all studies having a minimum 2-year follow-up, the failure rate ranged from 0% to 30% in the primary ACI group and 0% to 75% in the ACI after BMS group. Six of the studies 15,20,21,23,26,32 reported statistically significant (P < .05) increases in failure rate for patients undergoing prior BMS. One study 4 did not offer a comparison with control but reported a failure rate of 20%, consistent with other studies. The remaining 4 studies 29,31,33,41 did not find any statistically significant difference between groups.
Figure 2.
Failure rates at 2- to 5-year follow-up. *Statistically significant difference between primary ACI and ACI after BMS (P < .05). ACI, autologous chondrocyte implantation; BMS, bone marrow stimulation.
Figure 3.
Failure rates at >12 years’ follow-up. *Statistically significant difference between primary ACI and ACI after BMS (P < .05). ACI, autologous chondrocyte implantation; BMS, bone marrow stimulation.
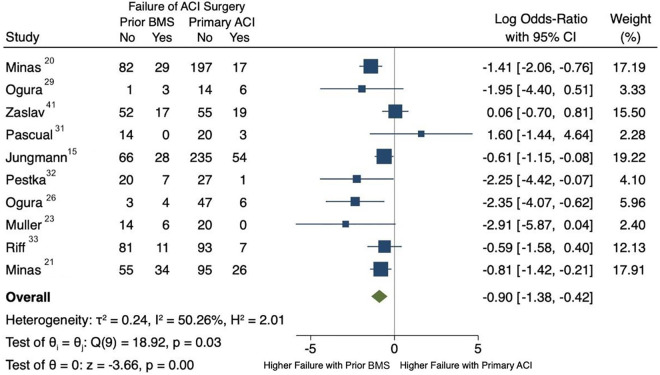
Overall, failure was observed in 26.4% of patients with previous BMS (139/527 patients) and in 14.8% of patients without prior BMS (139/942 patients). Meta-analysis demonstrated a higher failure rate in patients with a prior history of BMS (log odds ratio = –0.90; 95% confidence interval, –1.38 to –0.42) (Figure 4). Heterogeneity between studies was moderate, with an I 2 value of 50.26%. A funnel plot analysis was performed to assess bias, and all but 1 study 41 were within the established confidence limits (Figure 5).
Figure 4.
Forest plot. ACI, autologous chondrocyte implantation; BMS, bone marrow stimulation.
Figure 5.
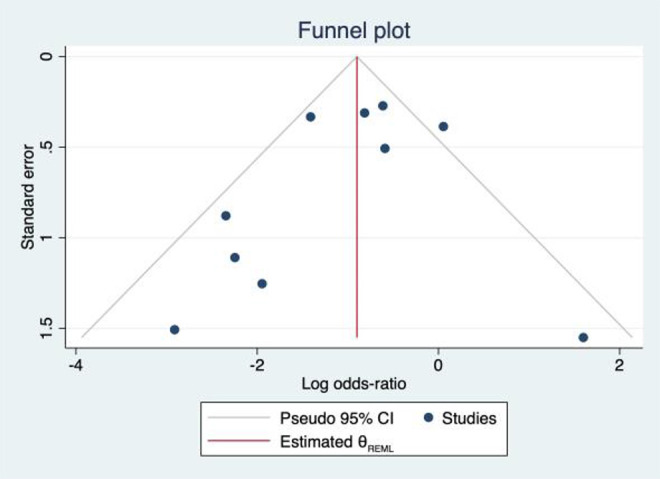
Funnel plot. REML, random-effects model.
ACI Techniques Used
Reflecting the time period over which these studies were published, a variety of ACI techniques were used in these studies. Five studies used only first-generation ACI technique with periosteal flaps. 20,21,29,31,41 One study 32 evaluated only a second-generation ACI technique with collagen membrane. One study 23 evaluated only a third-generation ACI technique with allogenic or autogenic stem cell–mediated cartilage regeneration. Three studies evaluated both first- and second-generation ACI techniques, 4,26,33 and 1 study 15 evaluated all 3 generations of ACI.
Patient-Reported Outcomes
All studies recorded PROs as outcome variables. Reporting of PROs can be seen in Table 3. Six of the 11 studies 21,23,31 -33,41 offered comparative analysis of PROs between the primary ACI group and ACI with prior BMS group. Four of the studies demonstrated no difference in PROs both in the primary ACI and ACI after BMS groups. 21,31,33,41 Two of the studies demonstrated significantly better PROs for patients undergoing primary ACI compared with ACI after prior BMS. 23,32
BMS Procedures Used
There was much heterogeneity in the reporting of the definition of BMS procedure among studies. Two studies reported that all prior BMS procedures were microfractures. 31,32 Three studies reported and specified a variety of techniques, including microfracture, abrasion arthroplasty, or drilling. 20,21,41 Six studies did not specify which BMS procedure their cohorts underwent prior to ACI 4,15,23,26,29,33 (Table 2).
Discussion
The purpose of this study was to evaluate outcomes of ACI after prior BMS relative to primary ACI, and we observed via pooled analysis that failure is significantly more likely for patients undergoing ACI after prior BMS. Using BMS as a first-line therapy for cartilage injury in the knee has been a common approach for many years likely because of the ease, familiarity, and low cost of the procedure. 17 However, the data presented in this review suggest patients who have cartilage lesions amenable to ACI may benefit more from primary ACI than ACI after failed BMS and that patients with failed BMS may have more predictable results with alternative treatment options such as osteochondral grafting when possible. 13,33 This observation of higher failure rates is likely due to changes in the subchondral bone, such as sclerosis, subchondral cysts, and intralesional osteophytes seen after microfracture that may inhibit proper healing of the ACI graft. 8,37 These histologic changes suggest that the milieu of a cartilage lesion after a BMS may in fact be detrimental to future ACI.
The goal of an ACI procedure is to restore the physiologic weightbearing surface of the joint, which requires not only a healthy articular surface but also healthy subchondral bone, and the 2 together are known as the osteochondral unit. 11 As such, a successful ACI relies on healing of the graft onto healthy subchondral bone. Given that the basis of BMS is to injure the subchondral bone lamellae to induce a healing response, it is thought that this injury may jeopardize future ACI graft healing potential. 18 Prior studies have demonstrated a 27% to 33% incidence of subchondral plate thickening and intralesional osteophytes after BMS, 16,22 and often subchondral bone defects >2 to 3 mm deep require filling before ACI transplantation; this may explain why higher failure rates are seen with patients having undergone prior BMS. 24 Interestingly, the finding of subchondral edema following ACI remains a controversial topic, and this should be an area of future research. It has been suggested that edema in the subchondral bone is part of the healing response of the ACI graft, 25 whereas others have suggested it may portend a worse prognosis. 18,24
Of the 11 studies presented in this review, 1 study 4 did not compare outcomes of ACI after BMS with a control group, such as primary ACI, and 4 studies 29,31,33,41 found no statistical difference between failure rates for ACI after BMS compared with primary ACI. One limitation that affected many of these studies on ACI, particularly with long-term follow-up data, was an underpowered sample size. The infrequency of osteochondral lesions that are indicated for ACI makes it difficult to collect data with high-quality, long-term follow-up, and this often results in studies that are underpowered. Another possible reason that some studies may not have demonstrated any difference between groups is that there is inherent selection bias in determining treatment for patients in nonrandomized study designs. Riff et al 33 compared primary and secondary ACI and found there was no difference in postoperative functional scores, subjective satisfaction, reoperation rate, or clinical failure. This was, however, a retrospective cohort study, so it is possible that careful patient selection for treatment with ACI after BMS contributed to the observed good outcomes. Further studies to identify potential factors for favorable ACI outcome after microfracture could help us better understand how to optimize patient outcomes. The heterogeneity both in ACI techniques and in definitions of failure is important to note when comparing outcomes in primary and secondary ACI literature.
It is also important to note that results after revision surgery in general will likely have a greater likelihood of failure in general, and it is not possible to clearly delineate the factors that cause treatment failure. Ogura et al 27 demonstrated this phenomenon in a study on revision ACI in which their analysis demonstrated failure rates of 30% to 50% at 5- and 10-year follow-up for revision ACI, which is inferior to that seen in primary ACI. 2,5,19 This has also been seen for other cartilage procedures in the knee, such as osteochondral allograft. 14 Given that ACI after a prior BMS represents a revision scenario, it is not surprising that the results of this paper demonstrated a similar trend to other revision cartilage restoration techniques, which suggests that a revision setting alone may contribute to increased failure rates. In addition to the revision setting, however, the alteration of the subchondral bone from BMS and other cartilage restoration techniques is what creates an unfavorable milieu for future surgery. 27
Another interesting finding in this review was regarding the heterogeneity of the definition of failure. Common definitions were any subsequent procedure that violated the subchondral bone, magnetic resonance imaging scan or arthroscopic evidence of graft delamination, and conversion to arthroplasty. However, some of the indications and definitions were less clear, such as failure of PROs to improve from baseline for >18 months, revision procedure for pain or discomfort, and revision cartilage repair. Although we cannot comment on the reason for failure in each case for comparison, 10 of the 11 studies were comparative cohort analyses, so the definitions of failure were evenly applied to both groups and did not introduce bias that would explain the higher failure rate in the patients with prior BMS. Despite heterogeneous definitions of failure among studies, the same definition of failure was applied to each patient in the primary ACI and ACI after BMS groups in each study.
These indications do not clearly define the patient population meeting criteria for “failure.” Furthermore, certain definitions of failure do not represent the clinical condition of all patients in the cohort of ACI recipients. For instance, for young, healthy patients undergoing ACI, using conversion to arthroplasty as a definition of failure may not be appropriate because they are extremely unlikely to reach this endpoint. Likewise, referring to any revision procedure as a failure may be too aggressive, especially for patients who do well after a simple revision procedure such as arthroscopic debridement. Though this heterogeneity makes it difficult to clearly define outcomes for the purposes of research, it does demonstrate the reality of “failure” being variably interpreted by both physician and patient. As such, it is important in practice that the surgeon and patient make their decision on an individualized patient basis depending on their expectations of improvement and recovery. Establishing clear and consistent clinical definitions of failure can be an important direction for future cartilage research studies.
In addition to overall increased rates of failure in the group undergoing ACI after BMS, 2 studies demonstrated additional variables in subgroup analysis as independent risk factors for failure. Minas et al 20 found that both complex and salvage defects were independent risk factors for failure at 30% and 24%, respectively, compared with 11% for the simple defects. In their study, complex defects were defined as multifocal lesions; single lesions >4 cm2; or lesions in the trochlea, tibia, or patella. Salvage defects were defined as bipolar lesions or any defects with signs of early arthritis. They did not find any differences in workers’ compensation (WC) status. The analysis by Riff et al, 33 however, demonstrated a higher rate of failure in the WC group undergoing ACI after BMS. They found a failure rate of 17% in the WC group compared with 6% for the non-WC group.
There are a variety of both patient-specific and lesion-specific risk factors that have been found to predispose patients to ACI graft failure. Patient-specific factors include increased age, female sex, and WC status. 19,30,33 Lesion-specific factors include complexity of lesion, history of prior surgeries of the affected joint, first-generation ACI technique, lesion size >4.5 cm2, and the failure to perform concomitant tibial tubercle osteotomy when indicated. 15,21,28,30 These risk factors have been described elsewhere in the literature, and given the heterogeneity of data reporting in the included studies, we could not adjust for all these factors in our review. However, given the results of the studies highlighted in this review, a history of prior BMS is another risk factor that should be added to the list of factors predisposing to ACI graft failure.
It is interesting to consider primary ACI versus BMS in the setting of cost-effectiveness because, although primary ACI may lead to a better outcome, it is not always feasible in an ability- or resource-limited setting. In a recent review of level 1 and 2 studies with 5-year follow-up, Aae et al 1 demonstrated that microfracture had greater cost-effectiveness when compared with ACI for focal chondral defects. 1 This was also supported by a review by Schrock et al, 34 who demonstrated that microfracture had the greatest cost-effectiveness for focal chondral defects compared with OATs and first- and second-generation ACI. However, they demonstrated that second-generation ACI had the greatest improvement in functional outcomes. These studies did not provide analysis beyond the midterm, however, which is when microfracture is most likely to fail, leading to increased costs in additional visits and invasive procedures. In a resource-limited setting, it may be acceptable to perform the BMS procedure before ACI, but this should warrant a frank discussion with the patient that it may not yield as promising a long-term solution. In a setting where resources and ability to perform ACI are not limited, it is our recommendation that primary ACI be performed instead of BMS given the results of this analysis.
Limitations
The limitations of this systematic review include only 11 studies meeting the inclusion criteria and the exclusion of all studies written in a non-English language. The majority of these studies were level 3 or 4 evidence, which subjects much of the data to the biases of case series studies. This includes lack of randomization and retrospectively collected data. Similarly, retrospective studies are subject to surgeon decision-making bias. It is important to note there was some overlap between cohorts by authors Minas et al 20,21 and Ogura et al 26,29 because they published on cohorts at different maturities. These data were stratified by follow-up time frame for failure rates; however, we were not able to completely exclude this overlap for the meta-analysis. In addition, there was significant heterogeneity in the populations and their procedures, such as BMS techniques and ACI generation, which makes it difficult to draw definitive comparisons among the studies as a whole. There was no way to control for concomitant procedures such as osteotomies and ligament reconstruction. It is possible that some amount of patient improvement in outcome in these studies is clouded by these concomitant procedures as well. The meta-analysis findings will be subject to this heterogeneity as well as the heterogeneity in definition of treatment failure; however, the pooled analysis still offers insight regarding the relative outcome of ACI with or without a history of prior BMS. We were not able to perform a meta-analysis on outcome scores or control for patient-specific factors in the meta-analysis of failure rates. Follow-up was robust, with a minimum of 2 years for all studies; however, greater follow-up duration would be ideal for evaluating outcomes from cartilage procedures. Finally, there was no way to perform subgroup analysis for ACI generation because we did not have granular patient data for some of the studies reporting multiple generations of ACI technology.
Conclusion
This systematic review demonstrated that failure rates were significantly higher for patients treated with ACI after a history of prior BMS relative to patients undergoing primary ACI without prior BMS. This finding has important implications when considering using BMS for defects that are amenable to cell-based restoration and when determining treatment options after failed BMS.
Footnotes
Final revision submitted March 16, 2021; accepted April 30, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: J.F. and J.Y. have received education payments from Evolution Surgical. A.L.Z. has received consulting fees from Stryker and hospitality payments from Arthrex and Zimmer. B.T.F. has received hospitality payments from Zimmer. C.B.M. has received royalties from Linvatec and consulting fees from Linvatec, Medacta, Stryker, Wright Medical, and Zimmer. D.L. has received grant support from Arthrex; education payments from Arthrex, Medwest, Smith & Nephew, and Evolution Surgical; and hospitality payments from Wright Medical. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Aae TF, Randsborg PH, Lurås H, Årøen A, Lian ØB. Microfracture is more cost-effective than autologous chondrocyte implantation: a review of level 1 and level 2 studies with 5 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1044–1052. doi:10.1007/s00167-017-4802-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aldrian S, Zak L, Wondrasch B, et al. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: a prospective follow-up at a minimum of 10 years. Am J Sports Med. 2014;42(11):2680–2688. doi:10.1177/0363546514548160 [DOI] [PubMed] [Google Scholar]
- 3. Bae DK, Song SJ, Yoon KH, Heo DB, Kim TJ. Survival analysis of microfracture in the osteoarthritic knee—minimum 10-year follow-up. Arthroscopy. 2013;29(2):244–250. doi:10.1016/j.arthro.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 4. Beck JJ, Sugimoto D, Micheli L. Sustained results in long-term follow-up of autologous chondrocyte implantation (ACI) for distal femur juvenile osteochondritis dissecans (JOCD). Adv Orthop. 2018;2018:7912975. doi:10.1155/2018/7912975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biant LC, Bentley G, Vijayan S, Skinner JA, Carrington RWJ. Long-term results of autologous chondrocyte implantation in the knee for chronic chondral and osteochondral defects. Am J Sports Med. 2014;42(9):2178–2183. doi:10.1177/0363546514539345 [DOI] [PubMed] [Google Scholar]
- 6. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–460. doi:10.1016/S0749-8063(97)90124-9 [DOI] [PubMed] [Google Scholar]
- 7. Davies-Tuck ML, Wluka AE, Wang Y, et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(3):337–342. doi:10.1016/j.joca.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 8. Demange MK, Minas T, von Keudell A, Sodha S, Bryant T, Gomoll AH. Intralesional osteophyte regrowth following autologous chondrocyte implantation after previous treatment with marrow stimulation technique. Cartilage. 2017;8(2):131–138. doi:10.1177/1947603516653208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fu FH, Zurakowski D, Browne JE, et al. Autologous chondrocyte implantation versus debridement for treatment of full-thickness chondral defects of the knee: an observational cohort study with 3-year follow-up. Am J Sports Med. 2005;33(11):1658–1666. doi:10.1177/0363546505275148 [DOI] [PubMed] [Google Scholar]
- 10. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986–1996. doi:10.1007/s00167-013-2676-8 [DOI] [PubMed] [Google Scholar]
- 11. Gomoll AH, Madry H, Knutsen G, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434–447. doi:10.1007/s00167-010-1072-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goyal D, Goyal A, Keyhani S, Lee EH, Hui JHP. Evidence-based status of second- and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy. 2013;29(11):1872–1878. doi:10.1016/j.arthro.2013.07.271 [DOI] [PubMed] [Google Scholar]
- 13. Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885–891. doi:10.1177/0363546514565770 [DOI] [PubMed] [Google Scholar]
- 14. Horton MT, Pulido PA, McCauley JC, Bugbee WD. Revision osteochondral allograft transplantations: do they work? Am J Sports Med. 2013;41(11):2507–2511. doi:10.1177/0363546513500628 [DOI] [PubMed] [Google Scholar]
- 15. Jungmann PM, Salzmann GM, Schmal H, Pestka JM, Südkamp NP, Niemeyer P. Autologous chondrocyte implantation for treatment of cartilage defects of the knee: what predicts the need for reintervention? Am J Sports Med. 2012;40(1):58–67. doi:10.1177/0363546511423522 [DOI] [PubMed] [Google Scholar]
- 16. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119–1125. doi:10.1016/j.joca.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 17. Lamplot JD, Schafer KA, Matava MJ. Treatment of failed articular cartilage reconstructive procedures of the knee: a systematic review. Orthop J Sports Med. 2018;6(3):1–10. doi:10.1177/2325967118761871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Madry H. The subchondral bone: a new frontier in articular cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):417–418. doi:10.1007/s00167-010-1071-y [DOI] [PubMed] [Google Scholar]
- 19. Martinčič D, Mekač J, Drobnič M. Survival rates of various autologous chondrocyte grafts and concomitant procedures: a prospective single-center study over 18 years. Cell Transplant. 2019;28(11):1439–1444. doi:10.1177/0963689719861922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902–908. doi:10.1177/0363546508330137 [DOI] [PubMed] [Google Scholar]
- 21. Minas T, Von Keudell A, Bryant T, Gomoll AH. The John Insall Award: a minimum 10-year outcome study of autologous chondrocyte implantation knee. Clin Orthop Relat Res. 2014;472(1):41–51. doi:10.1007/s11999-013-3146-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mithoefer K, Williams RJ III, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. J Bone Joint Surg Am. 2005;87(9):1911–1920. doi:10.2106/00004623-200509000-00002 [DOI] [PubMed] [Google Scholar]
- 23. Müller PE, Gallik D, Hammerschmid F, et al. Third-generation autologous chondrocyte implantation after failed bone marrow stimulation leads to inferior clinical results. Knee Surg Sports Traumatol Arthrosc. 2020;28(2):470–477. doi:10.1007/s00167-019-05661-6 [DOI] [PubMed] [Google Scholar]
- 24. Niemeyer P, Albrecht D, Andereya S, et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee. 2016;23(3):426–435. doi:10.1016/j.knee.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 25. Niethammer TR, Valentin S, Gülecyüz MF, et al. Bone marrow edema in the knee and its influence on clinical outcome after matrix-based autologous chondrocyte implantation: results after 3-year follow-up. Am J Sports Med. 2015;43(5):1172–1179. doi:10.1177/0363546515573935 [DOI] [PubMed] [Google Scholar]
- 26. Ogura T, Bryant T, Merkely G, Minas T. Autologous chondrocyte implantation for bipolar chondral lesions in the patellofemoral compartment: clinical outcomes at a mean 9 years’ follow-up. Am J Sports Med. 2019;47(4):837–846. doi:10.1177/0363546518824600 [DOI] [PubMed] [Google Scholar]
- 27. Ogura T, Bryant T, Merkely G, Mosier BA, Minas T. Survival analysis of revision autologous chondrocyte implantation for failed ACI. Am J Sports Med. 2019;47(13):3212–3220. doi:10.1177/0363546519876630 [DOI] [PubMed] [Google Scholar]
- 28. Ogura T, Bryant T, Mosier BA, Minas T. Autologous chondrocyte implantation for bipolar chondral lesions in the tibiofemoral compartment. Am J Sports Med. 2018;46(6):1371–1381. doi:10.1177/0363546518756977 [DOI] [PubMed] [Google Scholar]
- 29. Ogura T, Mosier BA, Bryant T, Minas T. A 20-year follow-up after first-generation autologous chondrocyte implantation. Am J Sports Med. 2017;45(12):2751–2761. doi:10.1177/0363546517716631 [DOI] [PubMed] [Google Scholar]
- 30. Pareek A, Carey JL, Reardon PJ, Peterson L, Stuart MJ, Krych AJ. Long-term outcomes after autologous chondrocyte implantation: a systematic review at mean follow-up of 11.4 years. Cartilage. 2016;7(4):298–308. doi:10.1177/1947603516630786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pascual-Garrido C, Slabaugh MA, L’Heureux DR, Friel NA, Cole BJ. Recommendations and treatment outcomes for patellofemoral articular cartilage defects with autologous chondrocyte implantation: prospective evaluation at average 4-year follow-up. Am J Sports Med. 2009;37(1)(suppl):33S–41S. doi:10.1177/0363546509349605 [DOI] [PubMed] [Google Scholar]
- 32. Pestka JM, Bode G, Salzmann G, Südkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40(2):325–331. doi:10.1177/0363546511425651 [DOI] [PubMed] [Google Scholar]
- 33. Riff AJ, Huddleston HP, Cole BJ, Yanke AB. Autologous chondrocyte implantation and osteochondral allograft transplantation render comparable outcomes in the setting of failed marrow stimulation. Am J Sports Med. 2020;48(4):861–870. doi:10.1177/0363546520902434 [DOI] [PubMed] [Google Scholar]
- 34. Schrock JB, Kraeutler MJ, Houck DA, McQueen MB, McCarty EC. A cost-effectiveness analysis of surgical treatment modalities for chondral lesions of the knee: microfracture, osteochondral autograft transplantation, and autologous chondrocyte implantation. Orthop J Sports Med. 2017;5(5):23259 67117704634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi:10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 36. Spahn G, Plettenberg H, Hoffmann M, Klemm HT, Brochhausen-Delius C, Hofmann GO. The frequency of cartilage lesions in non-injured knees with symptomatic meniscus tears: results from an arthroscopic and NIR- (near-infrared) spectroscopic investigation. Arch Orthop Trauma Surg. 2017;137(6):837–844. doi:10.1007/s00402-017-2672-4 [DOI] [PubMed] [Google Scholar]
- 37. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477–484. doi:10.1053/jars.2003.50112 [DOI] [PubMed] [Google Scholar]
- 38. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(suppl):S362–S369. doi:10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 39. Weber AE, Locker PH, Mayer EN, et al. Clinical outcomes after microfracture of the knee: midterm follow-up. Orthop J Sports Med. 2018;6(2):23259 67117753572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177–182. doi:10.1016/j.knee.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 41. Zaslav K, Cole B, Brewster R, et al. STAR Study Principal Investigators. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37(1):42–55. doi:10.1177/0363546508322897 [DOI] [PubMed] [Google Scholar]