Abstract
Early life adversity can disrupt development leading to emotional and cognitive disorders. This study investigated the effects of social isolation after weaning on anxiety, body weight and locomotion, and on extracellular dopamine (DA) and glutamate (GLU) in the nucleus accumbens (NAc) and their modulation by corticotropin releasing factor receptor 1. On the day of weaning, male rats were housed singly or in groups for 10 consecutive days. Anxiety-like behaviors were assessed by an elevated plus maze (EPM) and an open field test (OF). Neurotransmitter levels were measured by in vivo microdialysis. Single-housed rats spent less time, and entered more, into the closed arms of an EPM than group-housed rats. They also spent less time in the center of an OF, weighed more and showed greater locomotion. In the NAc, no differences in CRF, or in basal extracellular DA or GLU between groups, were observed. A depolarizing stimulus increased DA release in both groups but to higher levels in isolated rats, whereas GLU increased only in single-housed rats. Blocking CRF-R1 receptors with CP-154,526 decreased DA release in single-housed but not in group-housed rats. The corticotropin releasing factor receptor type 1 receptor antagonist also decreased GLU in group-housed animals. These results show that isolating adolescent rats increases anxiety, body weight and ambulation, as well as the sensitivity of dopaminergic neurons to a depolarizing stimulus. This study provides further evidence of the detrimental effects of social isolation during early development and indicates that dysregulation of the CRF system in the NAc may contribute to the pathologies observed.
Keywords: CRF, DA, early life stress, nucleus accumbens, young
Graphical Abstract
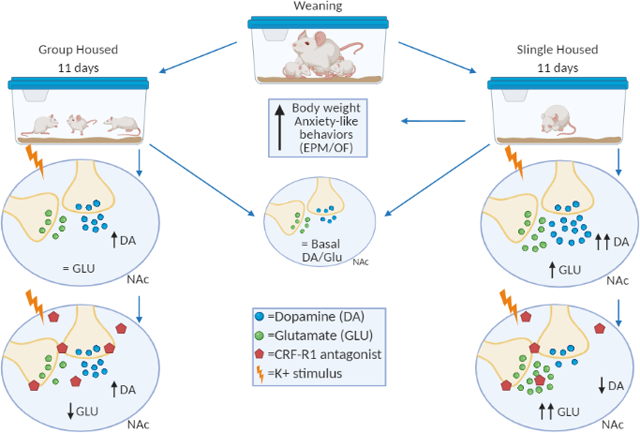
Following weaning, male rats are single or grouped-housed for 11 days. Single housed rats show increased anxiety and body weight. Microdialysis in the NAc revealed that extracellular DA and GLU were not affected. However, upon a depolarizing stimulus, DA increased to higher levels in single-housed rats. Depolarization induced an increase in GLU only in single-housed rats. Blocking CRFR1 increased DA in response to depolarization in group-housed rats, whereas single housed rats showed a decrease in basal and K+ DA in the presence of the CRFR1 antagonist. CRFR1 decreased GLU in group-housed animals. These results show that isolation during adolescence increases anxiety and the dopaminergic and glutamatergic response to a depolarizing stimulus. It also suggests that under normal housing conditions, CRF may act to curtail hyperresponsivity to a stimulus, an effect that is lost in animals exposed to chronic isolation during adolescence.
1 |. INTRODUCTION
Adolescence is a critical developmental stage that involves fine-tuning of the brain (Ernst et al., 2006). It is characterized by synaptic reorganization that ultimately defines many of our behavioral and emotional traits (Somerville et al., 2010). Adverse circumstances during this developmental period may exert neurochemical and behavioral changes that persist into adulthood (Shao et al., 2009; Vargas et al., 2016). For example, exposure to early life adversity, such as environmental stress, social instability and isolation, increases the risk to develop psychopathologies, like anxiety, depression, schizophrenia and drug addiction (Agid et al., 2000; Burke & Miczek, 2014; Lazarus & Cohen, 1977).
Anxiety is the mental disorder with the highest prevalence worldwide, affecting approximately 3.8% of the global population (Ritchie & Roser, 2019). It is characterized by a state of apprehension and hypervigilance in anticipation of perceived harmful events (Daviu et al., 2019). Anxiety disorders impair cognitive performance and impact quality of life (Xu et al., 2019). Loneliness is a major risk factor for the development of anxiety disorders (Mushtaq et al., 2014). Studies of social isolation in rodents are used as models to study the neurochemical sequelae of chronic early life adversity on the development of psychiatric disorders (Robbins et al., 1996). In this model, rats are isolated for several weeks and changes in behavior and brain neurochemistry studied subsequently, mainly during adulthood (Fone & Porkess, 2008; Grotewold et al., 2014). Results from these studies show dysregulation of the corticotropin releasing factor (CRF) system and development of anxiety-like behaviors (Ladd et al., 1996; Lukkes et al., 2008; Weiss et al., 2004).
Anxiety and stress share several neural underpinnings, among them activation of the hypothalamic-pituitary-adrenal (HPA) axis (Daviu et al., 2019). During the stress response, CRF, also known as corticotropin releasing hormone (CRH), is secreted by the paraventricular nucleus of the hypothalamus into the circulation and binds to receptors in the anterior pituitary to stimulate the secretion of adrenocorticotropic hormone that in turn induces the secretion of cortisol and corticosterone by the adrenal glands (Stevens & White, 2010). CRF is also synthetized by neurons of several other brain nuclei. CRF signaling is mediated through two types of G-protein coupled receptors, CRF-R1 and CRF-R2, that are widely expressed in brain areas like the ventral tegmental area (VTA), prefrontal cortex (PFC), dorsal raphe (DR) and nucleus accumbens (NAc) (Hauger et al., 2009; Henckens et al., 2016; Slater et al., 2016). There is compelling evidence indicating that CRF signaling is implicated in the regulation of anxiety, of cue-elicited motivation and of social behavior such as promoting social bonding (Deussing et al., 2010; DeVries, 2002; Lim et al., 2007). Several studies propose a direct relationship between the CRF system and stress (for review, see Backström & Winberg, 2013). There is evidence that CRF-R1 and CRF-R2 can participate in reactive or proactive stress coping strategies. However, the role of each receptor subtype in modulating the stress response in different brain regions remains unclear (Dedic et al., 2018; Deussing & Chen, 2018).
Early life stress induces changes in methylation patterns that can alter CRF expression. Many of these changes persist into adulthood and can mediate the observed increase in anxiety behavior (Elliott et al., 2010; Zhou & Fang, 2018). For example, prenatal stress was found to increase anxiety, decrease gene expression of CRF-R1, CRF-R2 and CRF-binding protein in the amygdala, and alter the response of the CRF family to acute stress in other brain areas such as the PVN (Zohar & Weinstock, 2011). Several studies suggest that CRF-R1 and CRF-R2 exert opposing effects on anxiety and dopamine (DA) release, with CRF-R1 inducing aversive and anxiogenic responses and CRF-R2 promoting anxiolytic and appetitive behaviors (Backström & Winberg, 2013). However, recent studies suggest that the role of these receptors subtypes can be complementary (Lemos et al., 2012) and can change according to the neuronal phenotype where they are located, the brain area under study and the condition under study (acute vs. chronic stress) (Dedic et al., 2018; Refojo et al., 2011; Slater et al., 2016; Williams et al., 2014).
The nucleus accumbens is crucial in cognition, reward, motivation, locomotion and addictive behaviors. It is finely tuned to the rewarding effects of social interactions during adolescence and plays a regulatory role in anxiety (Daviu et al., 2019; Somerville et al., 2010). The NAc receives CRF projections from the paraventricular nucleus of the hypothalamus and from the amygdala, brain regions associated with the stress response (Itoga et al., 2019).
The NAc can be divided into two main areas, the core and the shell. The NAc shell receives DA projections mainly from the VTA; it is associated with the mesocorticolimbic pathway and is involved in incentive motivation (Berridge, 2007). The NAc core is associated with the nigrostriatal pathway and plays a major role in reward predictive cues and locomotion (Morales & Berridge, 2020, Zahm, 1999).
There is evidence indicating that stress can modulate the amount of DA released in the NAc shell. Deutch and Cameron (1992) showed that acute restriction stress induced an increase of DA metabolites in the NAc shell but had no effect on the core. Likewise, Kalivas and Duffy (1995) showed that mild footshock stress increased extracellular DA levels in the shell of the NAc but had no effect on the core of the NAc. In addition, an increase in anxiety in socially isolated mice was associated with decreased expression of the transcription factor cAMP response element binding protein expression in the NAc shell (Wallace et al., 2009). As there is evidence that links stress with DA changes in the shell of the NAc, we targeted this area for study.
The role of CRF in modulating anxiety behavior may be mediated in part by modulating dopaminergic neurotransmission in the NAc. Indeed, injection of CRF into the NAc increases locomotor activity, a behavior dependent on accumbal DA (Holahan et al., 1997). In addition, an increase in DA release is observed after infusion of CRF into brain slices of the NAc (Lemos et al., 2012). It is possible that some of the negative effects of isolation on emotionality and motivated behaviors may be mediated by changes in CRF signaling in the NAc.
The purpose of this work was to address the effect of social isolation during adolescence on anxiety behavior and CRFergic modulation of the mesolimbic dopaminergic system. We used two experimental paradigms, male adolescent rats that were raised for 10 days under conditions of social isolation (single housed) and male adolescent rats that were group housed. Body weight and anxiety-like behaviors were assessed. Levels of CRF peptide in NAc tissue and of extracellular DA and glutamate (GLU) in the NAc were quantified. Furthermore, a CRF-R1 antagonist, CP-154,526, was used to analyze its effect on extracellular levels of DA and GLU in the NAc under both housing conditions.
2 |. MATERIALS AND METHODS
2.1 |. Animal group and treatment
Male Sprague–Dawley rats were obtained from the animal care facility of Pontificia Universidad Católica de Chile or ordered from Charles Rivers (University of Puerto Rico Medical Sciences Campus [UPR]). All animals were group housed from birth to weaning. At day 21 (day 23 at UPR), animals were weaned and divided into two sets: (a) group housed (2–3 rats per cage) and (b) single housed. Food and water were available ad libitum, the room was maintained at constant temperature (25°C) with a light/dark cycle of 12 hr (lights on at 07:00 a.m.). A different group of rats was used for each behavioral study, that is, two for the EPM and two for the open field studies. The behavioral tests were counter-balanced, and behavioral tests were conducted alternating isolated and grouped-housed individuals so that the time of testing was distributed equally among the comparison groups. The EPM test was conducted on PN day 32, and the open field test was conducted on PN day 33, as stated in Section 3. Six different groups of rats were used for the neurochemical studies (microdialysis and CRF determinations). Two groups were used to assess basal neurotransmitter levels, two other groups were used to assess neurotransmitter levels (brains were perfused with the CRF antagonist) and the remaining two groups were used to determine CRF levels by ELISA. All in all, 10 groups of rats (five experiments) were used for the entire study. All procedures were conducted according to institutional (Pontificia Universidad Católica de Chile and University of Puerto Rico Medical Sciences Campus), national and international guidelines (NIH Guide for the Care and Use of Laboratory Animals), and approved by the corresponding Institutional Animal Care and Use Committee.
2.2 |. Drugs
The CRF-R1 antagonist, CP-154,526, was purchased from Tocris Bioscience and dissolved in artificial cerebrospinal fluid (aCSF) at a concentration of 10 μM. The concentration of CP-154,526 was chosen according to previous work from our group (Sotomayor-Zarate et al., 2015).
2.3 |. Elevated plus maze
The elevated plus maze (EPM) is a behavioral assay validated in rodents to assess anxiety-related behavior. It is based on the rat’s aversion to open spaces and its tendency to remain near to, or touching, vertical surfaces (Pellow et al., 1985). Studies have shown that anxiogenic drugs decrease the time spent in open arms, while anxiolytic drugs increase the time spent in open arms (Biedermann et al., 2017). Our testing apparatus consisted of a plus-shaped custom-made apparatus with two 50 cm open arms and two 50 cm enclosed arms, each with an open roof. The apparatus is elevated 70 cm from the floor. An infrared video camera was placed above the maze. The camera was connected to a computer containing the ANY-maze™ software. At the beginning of the test, rats were placed at the junction of the open and closed arms, and the video tracking system was activated. The software automatically recorded the number of entries into the open and closed arms, as well as the time spent in each arm. Entry into an arm was defined as the time point when more than 95% of the length of the rat is in the arm. This was considered time zero. The test ended after 5 min. The amount of time spent in the open and closed arms, the number of entries into the open and closed arms, the time spent in the open arms relative to the closed arms and the total number of arm entries were measured. The less time spent, and the lower the number of entries into the open arms, the greater the anxiety.
2.4 |. Open field test
This test was originally developed in 1934 to measure emotionality in rats (Hall, 1934). It is used mainly to measure anxiety, exploratory behavior, risk taking behavior, thigmotaxis and effectiveness of anxiolytic drugs in rats (Treit & Fundytus, 1988). Anxiolytic drugs like benzodiazepines increase the amount of time the rat spends in the center of the open field, thus demonstrating their anxiolytic effect (Prut & Belzung, 2003). On the other hand, anxiogenic compounds, such as CRF, increase the amount of time spent in the periphery of the open field (Skutella et al., 1998). Its usefulness as a tool to measure anxiety has been the subject of debate, and several studies argue that it is more suited for measuring locomotor activity (Seibenhener & Wooten, 2015). Nonetheless, it is still used in conjunction with the EPM as a confirmatory behavioral assay for anxiety.
Ten locomotor activity chambers (Versamax™ system) were used to measure open field behavior. These chambers are made from clear acrylic (42 × 42 × 30 cm), with 16 equally spaced (2.5 cm) infrared beams across the length and width of the cage at a height of 2 cm from the cage floor (horizontal beams). All beams are connected to a Data Analyzer that sends information to a personal computer that displays beam data through a Windows-based program (Versadat). Animals were placed in the activity cage and allowed to roam freely for 10 min. The breaking of infrared beams determined the position of the rats in the activity cage. The amount of time spent in the center of the cage versus the amount spent at the periphery was compared, as well as the total distance travelled. Animals that spent less time in the center of the cage were considered to be more anxious.
2.5 |. Measurement of CRF
Eleven days after weaning (PND 31), a group of single- and group-housed rats were anesthetized with chloral hydrate (400 mg/kg), decapitated, the brain removed and the NAc micro-punched from coronal sections. The tissue was homogenized in 1 × RIPA lysis buffer (Millipore) and 1 × Complete Mini (Roche), sonicated at three cycles of 10 s and agitated at 4°C for 30 min. Finally, tissues were centrifuged at 4°C for 30 min at 15,000 RPM and proteins quantified using the micro-BCA kit (Thermo Scientific, Pierce) according to manufacturer specifications. CRF was quantified with an ELISA kit (Peninsula Laboratories) using protocol III according to manufacturer specifications.
2.6 |. Microdialysis
To assess extracellular neurotransmitter levels in the NAc, in vivo microdialysis was performed at PND 31 in two groups of rats: single housed and group housed. Rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and maintained with a continuous flow (1.5–4.5 μl/min) of chloral hydrate (8% w/v). The rat’s temperature was kept at 37°C with a heating pad. Microdialysis probes (MAB 2.14.2, cut-off 35 KDa, Microbiotech) were placed in the NAc using the following coordinates: AP: 1.3 mm, ML: 1.3 mm and DV:8.5 mm, at an angle of 20° from Bregma (Figure 1a). The probe was perfused with aCSF buffer, composed of 120 mM NaCl, 2.4 mM KCl, 0.9 mM NaH2PO4, 1.4 mM Na2HPO4 and 1.2 mM CaCl2 (pH 7.40). To investigate the effect of a depolarizing stimulus on extracellular DA and GLU levels in the NAc, potassium was added to the microdialysis probe (Girault et al., 1986; Moghaddam et al., 1990). For this procedure, the NAc was perfused with aCSF containing 70 mM KCl and 52.5 mM NaCl. The probe was placed in the NAc, stabilized over 90 min and then dialysate samples were collected every 10 min. K+-aCSF was applied, in every microdialysis experiment, between the 6th and 7th dialysate sample as a depolarizing stimulus. In the experiment with 10 μM CP-154,526, the antagonist was applied into the NAc after the 3rd dialysate. At the end of the experiments, animals were decapitated and their brains removed to assess probe localization.
FIGURE 1.
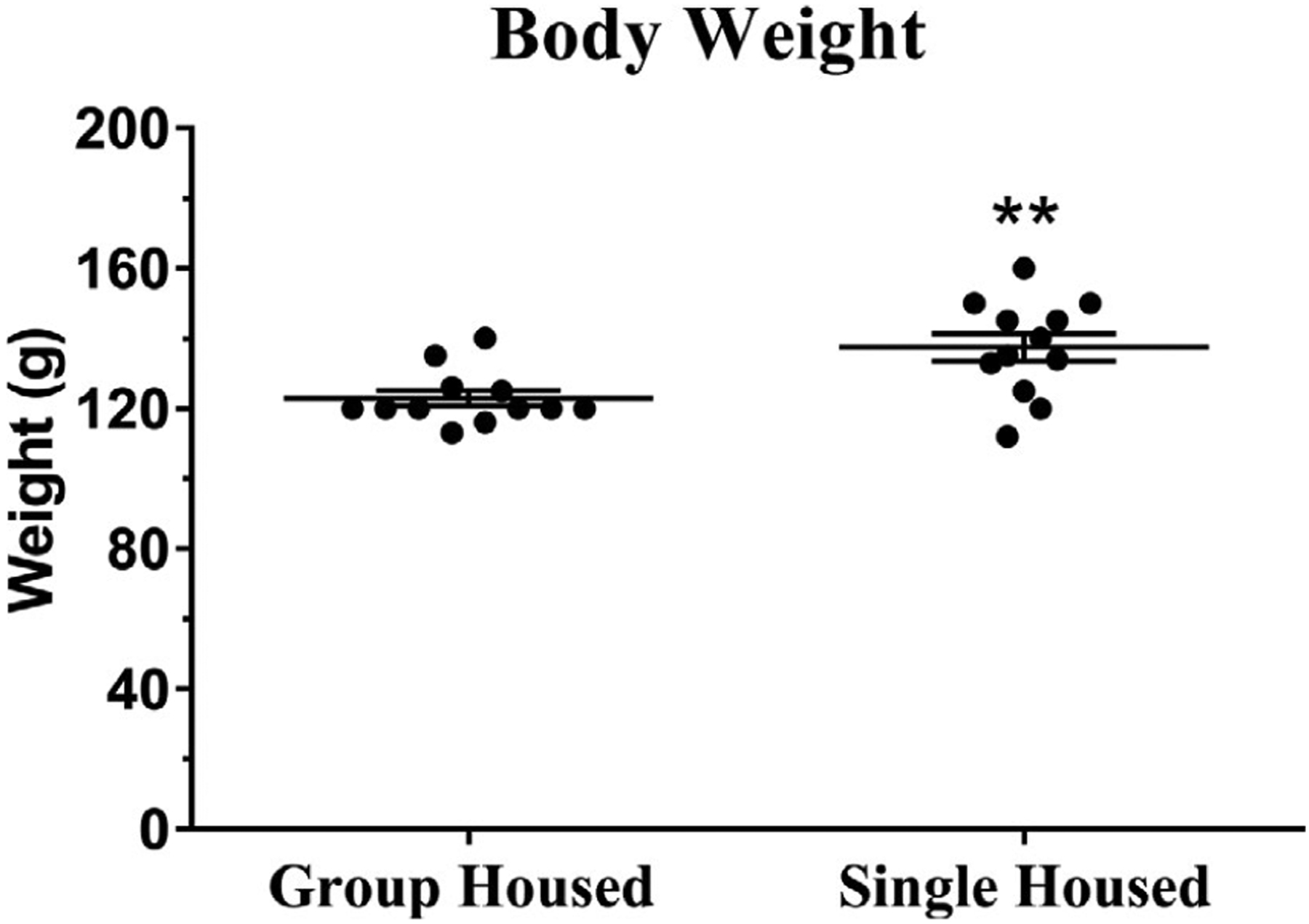
Body weight of male rats after 10 days of grouped or single housing. Animals were weighed on PND 31, after the isolation period and before any experimental manipulation. The body weight of single-housed rats was greater than that of group-housed rats. Number of animals = 12 per group. **Indicates significantly different from group-housed rats; see Table 1 for statistical tests and values
2.7 |. Analysis of dialysates by HPLC
Dialysate samples were analyzed by high performance liquid chromatography (HPLC). DA was detected with an electrochemical detector. The mobile phase was 0.1 M NaH2PO4, 0.1 mM EDTA, 1.7 mM octanesulfonic acid and 7% v/v CH3CN (pH 2.5). Glutamate was detected with a fluorometric detector. To this end, 10 μl of dialysate was mixed with 10 μ of ddH2O, 4 μl of borate buffer (pH 10.8) and 4 μl of fluorogenic reagent (20 mg of ortho-phthaldehyde and 10 μl 2-Mercaptoethanol in 5 ml of absolute ethanol). A total of 20 μl of the mixture was injected into the HPLC. The mobile phase was 0.1 mM NaH2PO4 and 23.5% v/v CH3CN (pH 5.7). The first three dialysates were used as the baseline to calculate the percent change from baseline in every sample.
2.8 |. Confirmation of probe localization
At the end of the microdialysis experiments, animals were euthanized, brains removed, placed in 4% paraformaldehyde for 48 hr, washed in PBS and placed in 25% sucrose for 2 days. Brains were then stored at −70°C and subsequently sliced at 20 μm in a cryostat. Brain slices were mounted on a glass slide and stained with cresyl violet (Figure 1b) to confirm the placement of the microdialysis probe. Data obtained from animals where the probe was not in the NAc were not used in the study.
2.9 |. Statistical analysis
The statistical analysis used varied according to the data evaluated. Body weight (Figure 2), EPM (Figure 3), open field (Figure 4a and b), CRF content (Figure 7), extracellular DA in NAc (Figure 5a) and extracellular GLU in NAc (Figure 6a) data were analyzed with an unpaired Student’s t test. K+ stimulated (Figures 5b and 6b) DA and GLU were analyzed with a two-way ANOVA, followed by Sidak post-hoc tests. The effects of the CRF-R1 antagonist CP-154,526 on extracellular DA and GLU in the NAc (Figures 5c,d and 6c,d) were combined and analyzed using a 3-way ANOVA. Statistical analysis was performed using GraphPad Prism software (versions 7 and 8) and JMP (version 10). An alpha level of p < 0.05 was considered statistically significant. Detailed statistical tests and values are included in Tables 1–3, as well as in Section 3.
FIGURE 2.
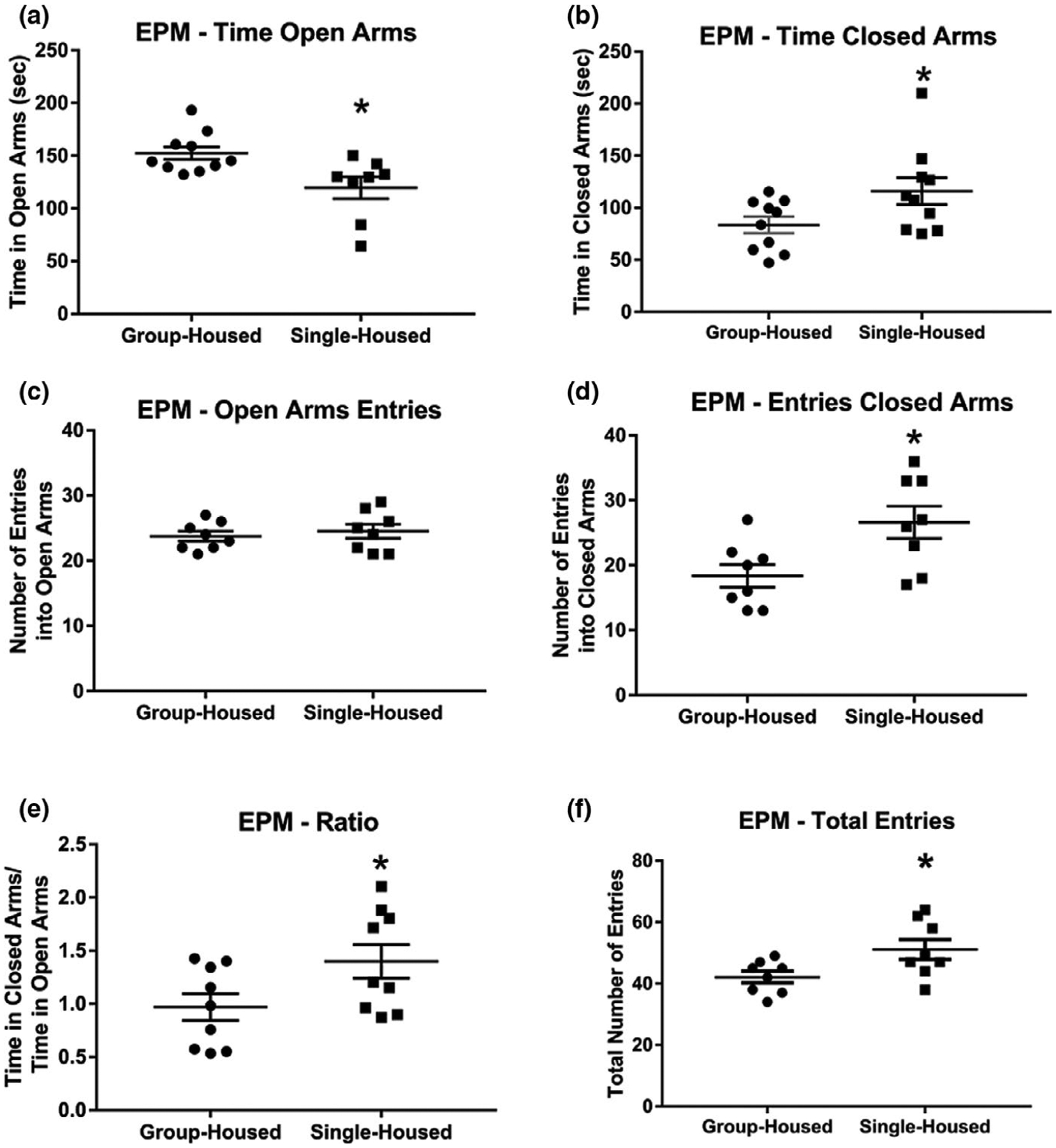
Elevated plus maze test results of group- and single-housed rats. (a) After 10 days of single housing (PN 22–31), isolated rats spent less time in the open arms of an EPM, (b) more time in the closed arms of an EPM, (c) showed no differences in the number of open arm entries, (d) made more entries into the closed arms of an EPM, (e) spent more time in closed versus open arms of the EPM and (f) had more total entries into both arms of an EPM than group-housed rats. Number of animals = 10 per group. *Indicates significantly different from group-housed rats; for complete statistical values, see Table 1
FIGURE 3.
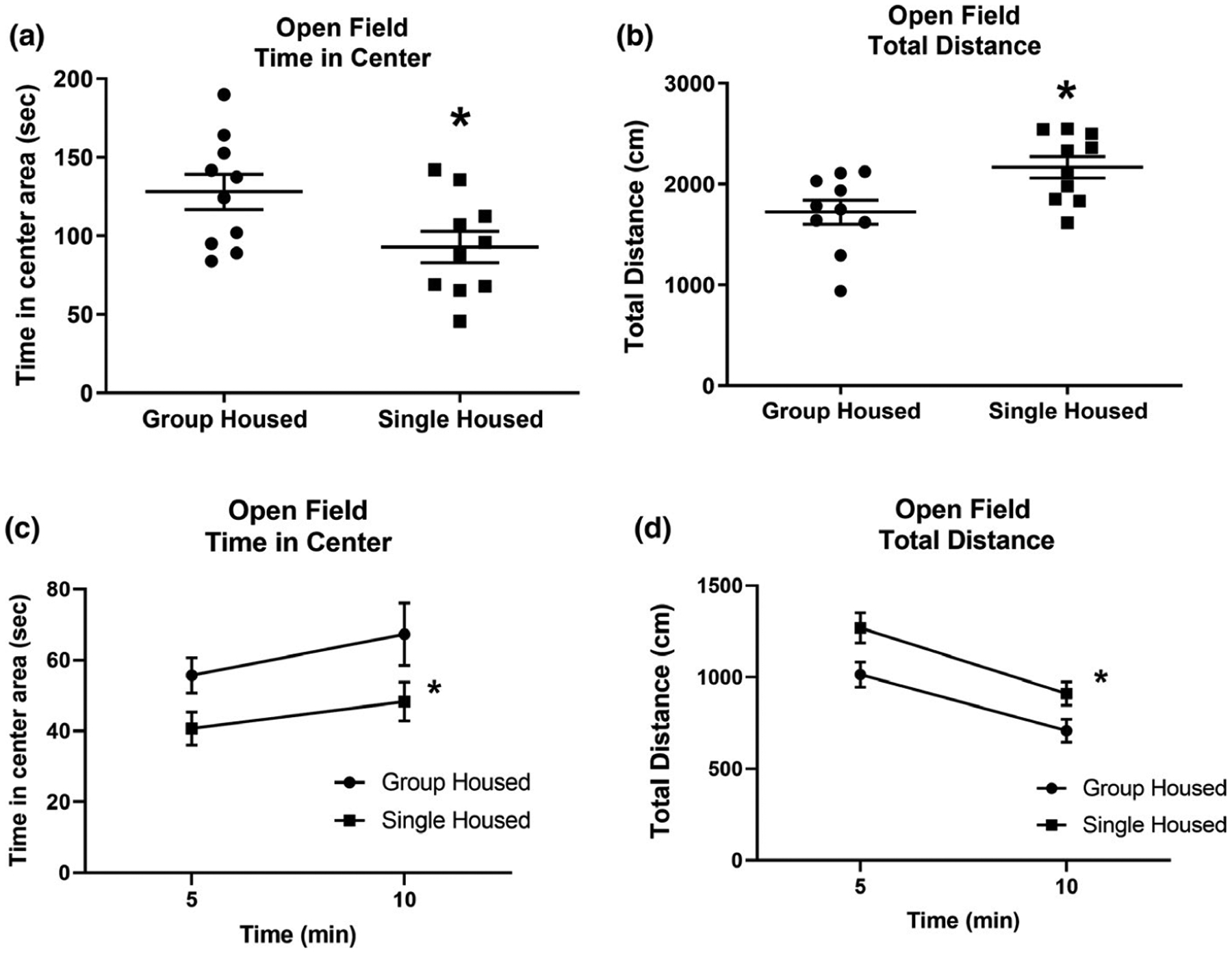
Open field behavior of group- and single-housed rats. (a) After 11 days of single housing (PN 22–32), rats spent less time in the center area, and (b) displayed greater ambulation than group-housed rats. (c and d) This difference was consistent throughout the open field test. Number of animals = 10 per group. *Indicates significantly different from group-housed rats; for statistical values, see Table 1 and Section 3
FIGURE 4.
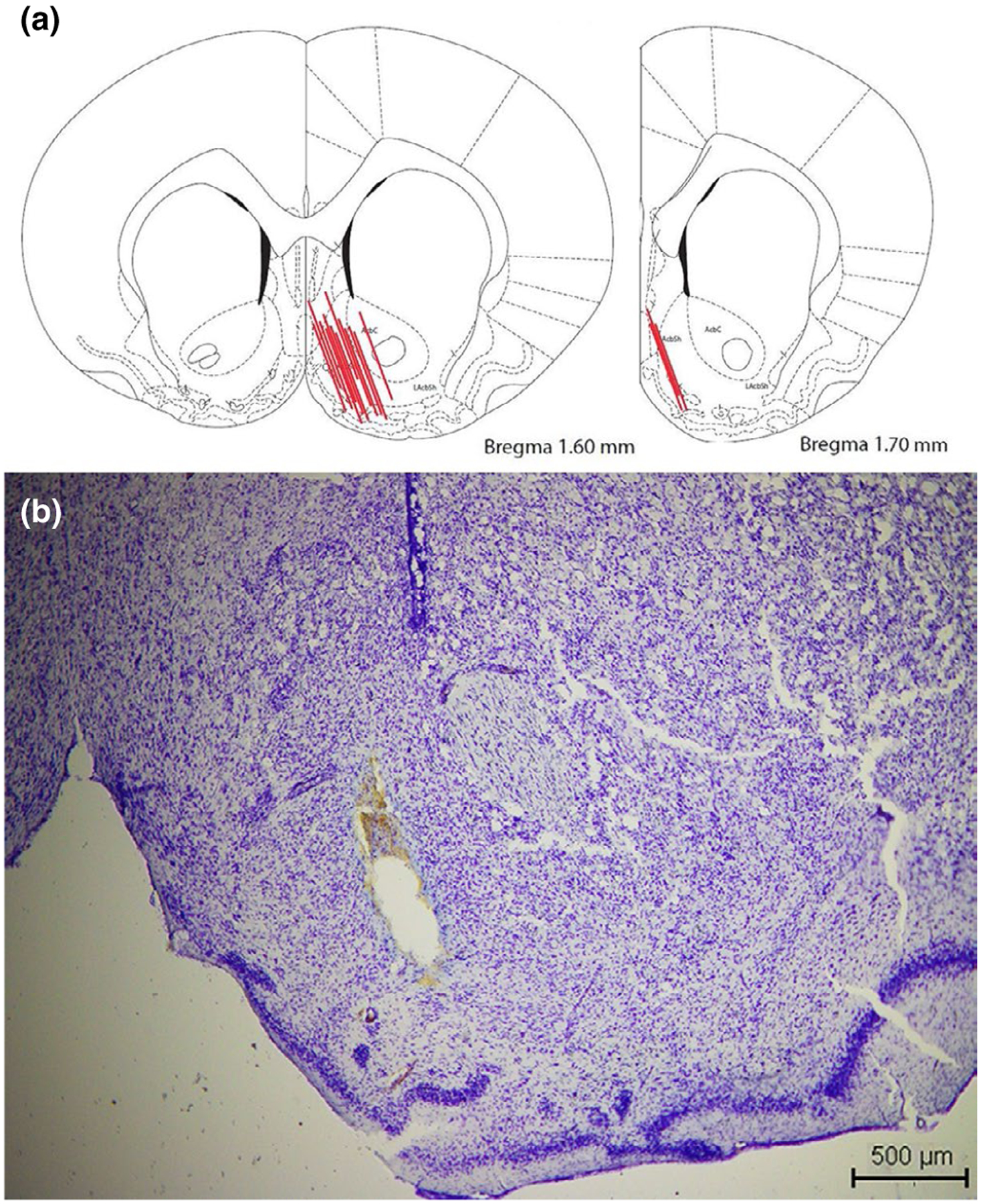
Anatomical localization of the microdialysis probes. (a) Representation of a rat brain coronal section and the position of the NAc microdialysis probes of rats included in this study. (b) Tissue damage by probe placement in the NAc. Scale: 500 μm (scheme modified from Paxinos & Watson, 2005)
FIGURE 7.
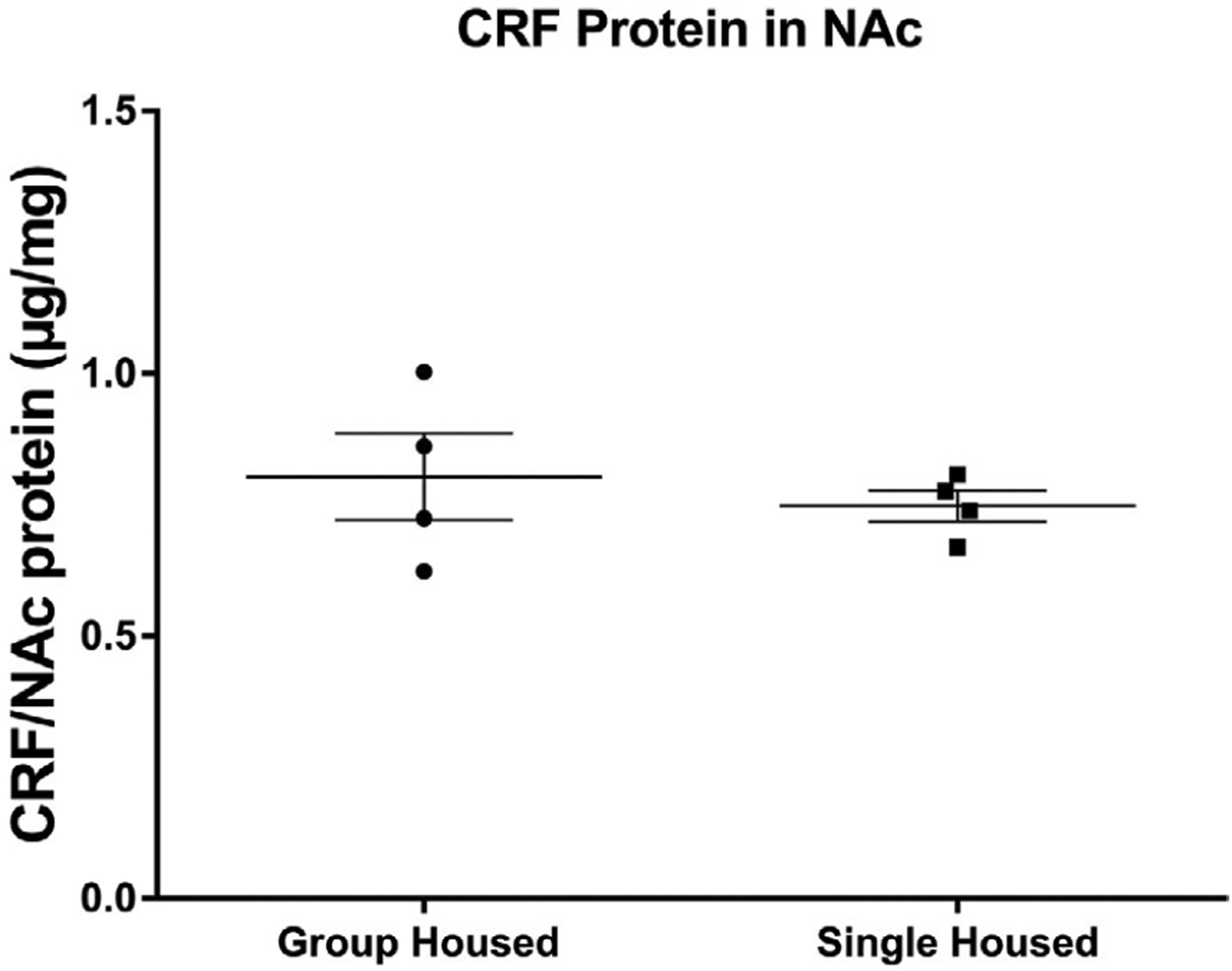
Amount of CRF in the nucleus accumbens of single and group-housed rats. After 10 days of isolation, a separate group of rats was euthanized, the nucleus accumbens dissected and homogenized, and levels of CRF measured by ELISA. CRF in the NAc did not differ between single- and group-housed rats. Data presented as mean ± SEM, n = 4. CRF values were normalized with respect to total NAc proteins (see Table 1 for statistical values)
FIGURE 5.
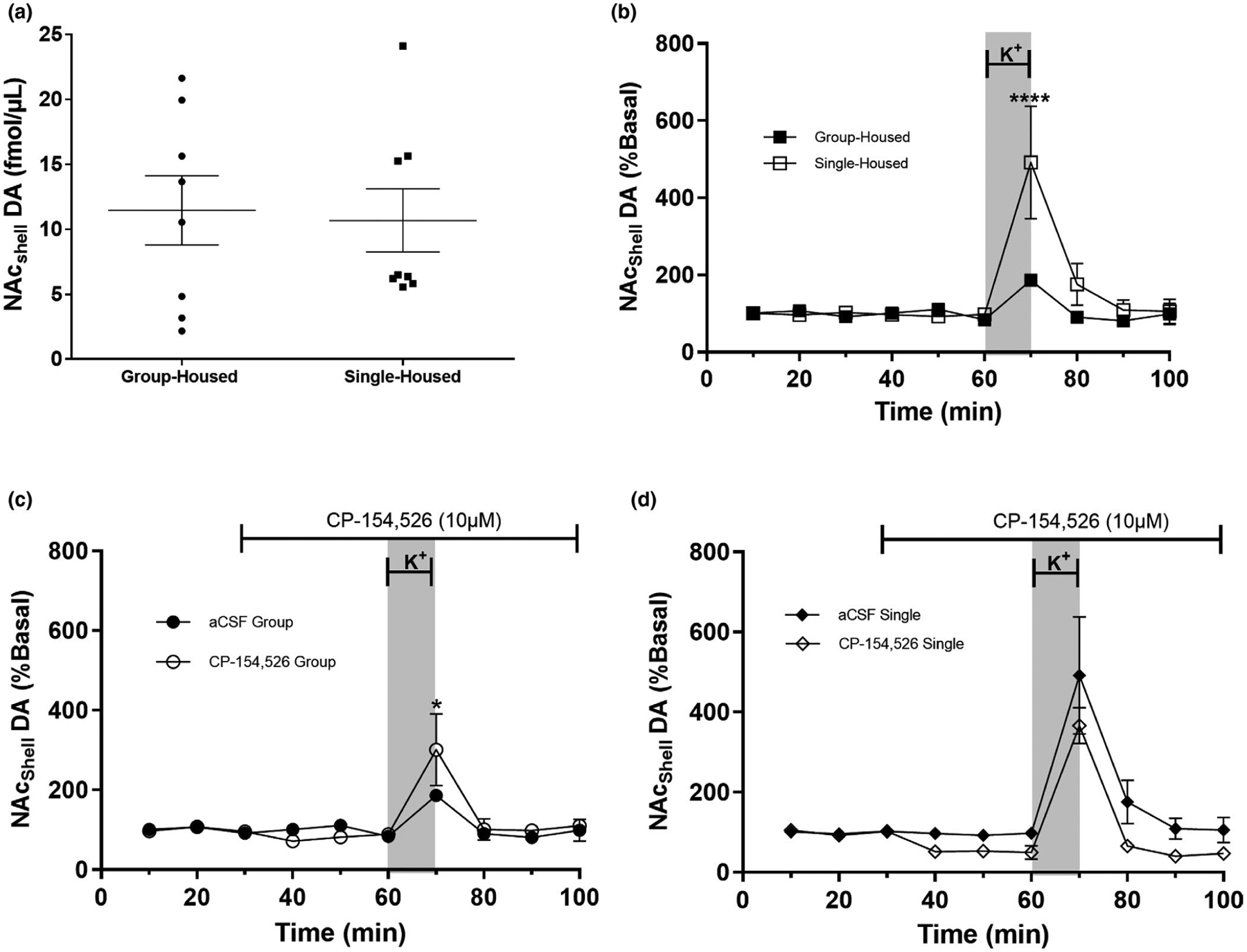
Extracellular dopamine in the NAc of single- and group-housed rats in the presence or absence of the CRF-R1 antagonist CP-154,526. After 11 days of single or group housing, extracellular DA in the NAc was assessed by microdialysis. (a) Basal extracellular DA was not affected by housing conditions (n = 8 per group). (b) Perfusion of the NAc shell with KCl (70 mM KCl) increased extracellular DA in both groups; this increase was significantly higher in single-housed rats (n = 5 per group). (c and d) Blocking CRF-R1 receptors in grouped-housed rats exacerbated the extracellular dopaminergic response to a depolarizing stimulus (n = 5 per group), an effect not observed in single-housed rats. Blocking CRF-R1 receptors in single-housed rats decreased extracellular dopamine (n = 5 per group), an effect not observed in group-housed rats. aCSF group (n = 5 per group), CP-154,526 group (n = 3 per group). For panels b, c and d, data are presented as mean ± SEM. For panels c and d, the gray bar represents the depolarizing stimulus (70 mM KCl) between 60 and 70 min. The line on top of the graph (between minutes 30 and 100) represents perfusion of the CRF-R1 antagonist CP-154,526. Data in Figure 5b–d were normalized to basal levels in each animal. Figure 5a: p = 0.8340 (Table 1); Figure 5b: ****indicates significantly different (Treatment: p = 0.0146, Time p = 0.0001, Interaction p = 0.0015. (For details, see Tables 1 and 2) In group-housed rats CP-154,256 induced an increase in the dopaminergic response to a depolarizing stimulus (see Table 2). Figure 5c and d: An interaction between housing and CP-154,526 was found (p = 0.0330), indicating that the response to the CRF antagonist varied depending on the housing conditions. The variable Time also varied among the groups (Time = 0.0118), indicating that the effect of CP-154,526 varied across the different time points (see Table 3 for statistical values)
FIGURE 6.
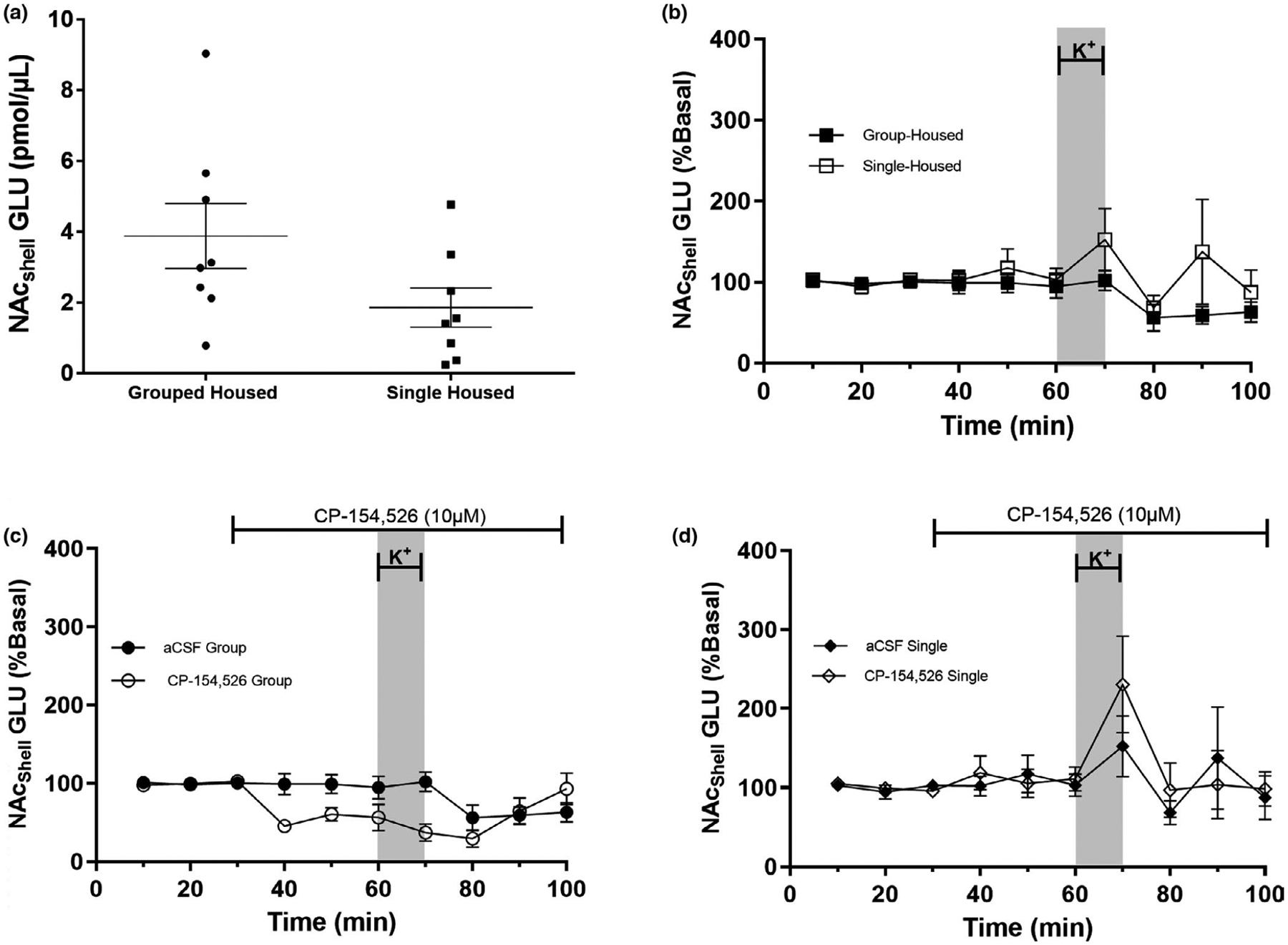
Extracellular glutamate in the NAc of single and group-housed rats in the presence or absence of the CRF-R1 antagonist CP-154,526. After 11 days of single or group housing, extracellular glutamate in the NAc was assessed by microdialysis. (a) Basal extracellular GLU was not affected by housing conditions (n = 8 per group). (b) The effect of KCl on extracellular GLU in the NAc shell (70 mM KCl) varied depending on housing conditions; it caused an increase in GLU in single-housed rats but had no effect on group-housed rats (n = 5 per group).(c and d) Perfusion of the NAc with CP-154,526 decreased extracellular GLU in group-housed rats, an effect not observed in single-housed rats. Perfusion with KCl (70 mM) in the presence of CP-154,526 induced an increase in GLU in single-housed but not in group-housed rats, aCSF group (n = 5) and CP-154,526 group (n = 3). For panels (b, c and d), data are presented as mean ± SEM. The gray bar represents the depolarizing stimulus (70 mM KCl) between 60 to 70 min. The line on top of the graph (between minutes 30 and 100) represents perfusion of the CRF-R1 antagonist CP-154,526. Data in Figure 6b–d were normalized to basal levels in each animal. Figure 6a: p = 0.0799 (Table 1); Figure 6b: Treatment p = 0.0436 (Table 1); Figure 6c and d: Results from 3-way RM ANOVA: Housing p = 0.0129, Time p = 0.0261, Time × Housing p = 0.0385 and Time × Housing × CP-154,256 p = 0.0161 (see Table 3 for statistical values details)
TABLE 1.
Statistical values for data analyzed by Student t test and 2-way ANOVA
| Figure | Variables | Statistics | Significance | p value |
|---|---|---|---|---|
| Figure 2—Body weight | Body Wt × Housing | Student t test | t = 3.1838 | 0.0043* |
| Figure 3a—EPM | Time Open × Housing | Student t test | t = 2.836, df = 18 | 0.0119* |
| Figure 3b—EPM | Time Closed × Housing | Student t test | t = 2.1443, df = 18 | 0.0460* |
| Figure 3c—EPM | Entries Open × Housing | Student t test | t = 0.5684, df = 18 | 0.5788 |
| Figure 3d—EPM | Entries Closed × Housing | Student t test | t = 2.703, df = 18 | 0.0172* |
| Figure 3e—EPM | EPM Ratio | Student t test | t = 2.127, df = 18 | 0.0493* |
| Figure 3f—EPM | EPM Total entries | Student t test | t = 2.397, df = 18 | 0.0311* |
| Figure 4a—OF center | Time Ctr × Housing | Student t test | t = 2.335, df = 18 | 0.0313* |
| Figure 4b—OF distance | Tot Distance × Housing | Student t test | t = 2.782, df = 18 | 0.0123* |
| Figure 5a–DA in NAc | DA in NAc × Housing | Student t test | t = 0.2135, df = 14 | 0.8340 |
| Figure 5b—K+ stim DA | Treatment | 2-way ANOVA | F(1–80) = 6.235 | 0.0146* |
| Time | F(9–80) = 8.211 | 0.0001* | ||
| Interaction | F(9–80) = 3.385 | 0.0015* | ||
| Figure 6a—Microdialysis | GLU in NAc × Housing | Student t test | t = 1.888, df = 14 | 0.0799 |
| Figure 6b—Microdialysis | Treatment | 2-way ANOVA | F(1–80) = 4.204 | 0.0436* |
| Time | F(9–80) = 1.373 | 0.2145 | ||
| Interaction | F(9–80) = 0.7439 | 0.6677 | ||
| Figure 7—CRF in NAc | CRF × Housing | Student t test | t = 0.6303, df = 6 | 0.5517 |
Single-housed rats weighed more.
TABLE 3.
Statistical values obtained from the 3-way repeated measures ANOVA for Figure 5c and d (dopamine) and 6c and d (glutamate)
| Analysis | Value | Exact F | Num DF | DenDF | Prob > F | |
|---|---|---|---|---|---|---|
| DA 40–100 | ||||||
| Between | All | 0.9098 | 3.6391 | 3 | 12 | 0.0448* |
| Intercept | 16.1377 | 193.6523 | 1 | 12 | <0.0001 | |
| Housing | 0.0766 | 0.9189 | 1 | 12 | 0.3567 | |
| CP-154,256 | 0.2099 | 2.5191 | 1 | 12 | 0.1385 | |
| Housing × CP | 0.4833 | 5.8001 | 1 | 12 | 0.0330* | |
| Within | All (Roy’s Max Root) | 3.3337 | 5.0006 | 6 | 9 | 0.0160* |
| Time | 5.8165 | 6.7859 | 6 | 7 | 0.0118* | |
| Time × Hous | 0.9485 | 1.1066 | 6 | 7 | 0.4425 | |
| Time × CP | 0.5893 | 0.6876 | 6 | 7 | 0.6679 | |
| Time × Hous × CP | 1.5890 | 1.8539 | 6 | 7 | 0.2190 | |
| DA 40–60 | ||||||
| Between | All | 1.6472 | 6.5888 | 3 | 12 | 0.0070* |
| Intercept | 33.6560 | 403.8719 | 1 | 12 | <0.0001* | |
| Housing | 0.3265 | 3.9182 | 1 | 12 | 0.0712 | |
| CP-154,256 | 1.2123 | 14.5481 | 1 | 12 | 0.0025* | |
| Housing × CP | 0.2130 | 2.5566 | 1 | 12 | 0.1358 | |
| Within | All (Roy’s Max Root) | 0.5033 | 2.0135 | 3 | 12 | 0.1658 |
| Time | 0.0820 | 0.4511 | 2 | 11 | 0.6482 | |
| Time × Hous | 0.1516 | 0.8341 | 2 | 11 | 0.4600 | |
| Time × CP | 0.1682 | 0.9254 | 2 | 11 | 0.4252 | |
| Time × Hous × CP | 0.2737 | 1.5055 | 2 | 11 | 0.2643 | |
| Glut 40–100 | ||||||
| Between | All | 0.7434 | 2.9738 | 3 | 12 | 0.0743 |
| Intercept | 10.5268 | 126.3224 | 1 | 12 | <0.0001* | |
| Housing | 0.7083 | 8.5005 | 1 | 12 | 0.0129* | |
| CP-154,256 | 0.0129 | 0.1546 | 1 | 12 | 0.7010 | |
| Housing × CP | 0.0126 | 1.5093 | 1 | 12 | 0.2428 | |
| Within | All (Roy’s Max Root) | 7.0672 (ap) | 10.6009 | 6 | 9 | 0.0012* |
| Time | 4.3122 | 5.0309 | 6 | 7 | 0.0261* | |
| Time × Hous | 3.6935 | 4.3092 | 6 | 7 | 0.0385* | |
| Time × CP | 0.4979 | 0.5809 | 6 | 7 | 0.7374 | |
| Time × Hous × CP | 5.1888 | 6.0536 | 6 | 7 | 0.0161* | |
| GLU 40–60 | ||||||
| Between | All | 0.8780 | 3.5186 | 3 | 12 | 0.0490* |
| Intercept | 16.9956 | 203.9481 | 1 | 12 | <0.0001* | |
| Housing | 0.5639 | 6.7672 | 1 | 12 | 0.0232* | |
| CP-154,256 | 0.1895 | 2.2735 | 1 | 12 | 0.1575 | |
| Housing × CP | 0.2828 | 3.3937 | 1 | 12 | 0.0903 | |
| Within | All (Roy’s Max Root) | 0.2951 | 1.1805 | 3 | 12 | 0.3581 |
| Time | 0.0150 | 0.0829 | 2 | 24 | 0.9210 | |
| Time × Hous | 0.0668 | 0.3676 | 2 | 24 | 0.7006 | |
| Time × CP | 0.0231 | 0.1269 | 2 | 24 | 0.8821 | |
| Time × Hous × CP | 0.2782 | 1.5304 | 2 | 24 | 0.2592 | |
A three way MANOVA indicates that extracellular DA levels in the NAc are significantly different between single-housed and group-housed animals. DA levels were also different between animals that received the CRF antagonist and those that received the vehicle.
3 |. RESULTS
3.1 |. Social isolation increases body weight
On the day of dialysis, prior to the experiments (day 31), body weight of rats was assessed. The average weight of group-housed rats was 122.9 ± 2.2 g, whereas single-housed rats weighed 137.4 ± 4.0 g (Figure 2). Statistical comparisons between groups reveal that single-housed rats had a higher body weight than those that were group housed. Data were analyzed by a non-paired Student’s t test. t = 3.1838; p = 0.0043.
3.2 |. Social isolation increases anxiety-like behavior
On day 32, rats were tested in the EPM. Single-housed rats spent less time in the open arms of the EPM than group-housed rats (Figure 3a). No differences were observed in open arm entries between groups (Figure 3c) Single-housed rats spent more time and made more entries, into the closed arms of the EPM than group-housed rats (Figure 3b and d). Social isolation also increased the ratio of the time spent in the closed arms versus open arms of the EPM (Figure 3e). The total number of entries into open and closed arms of the EPM also increased (Figure 3f). These results show that single housing increases anxiety in rodents. Data were analyzed with a non-paired Student’s t test comparing single-with group-housed animals. Figure 3a: t = 2.836, p = 0.0119; Figure 3b: t = 2.1443, p = 0.046; Figure 3c: t = 0.5684, p = 0.5788; Figure 3d: t = 2.703, p = 0.0172; Figure 3e: t = 2.127, p = 0.0493; Figure 3f: t = 2.397, p = 0.0311.
3.3 |. Social isolation increases ambulation
On day 33, rats were tested in an open field. Single-housed rats spent less time in the center of an open field (Figure 4a and c) and ambulated more (Figure 4b and d) than group-housed rats. These results afford proof that social isolation increases anxiety and locomotor activity in rats. Data from Figure 4a,b were analyzed with a non-paired Student’s t test comparing single- with group-housed animals: Figure 4a: t = 2.335, p = 0.0313; Figure 4a: t = 2.782, p = 0.0123. Data from Figure 4c,d were analyzed by linear regression comparing single- and group-housed rats. Figure 4c: unequal slopes: F = 0.3289, p = 0.5699; unequal intercepts: F = 10.101, p = 0.00299; Figure 4d: unequal slopes: F = 0.1394, p = 0.711; unequal intercepts: F = 10.9053, p = 0.00213.
3.4 |. Social isolation does not affect basal dopamine or glutamate extracellular levels in the NAc
To address if social isolation during adolescence alters dopaminergic or glutamatergic extracellular levels, microdialysis was used to obtain dialyzed samples of the NAc of group- and single-housed animals. The data obtained were normalized relative to the percent change from basal levels to reduce data variability. Our data from postnatal day 31 show no significant differences in extracellular basal DA between group-housed and single-housed rats (Figure 5a). Extracellular basal GLU levels also did not vary between groups (Figure 6a). Data in Figures 5a and 6a were analyzed by non-paired Student’s t test (Figure 5a: t = 0.2135, p = 0.8340; Figure 6a: t = 1.8881, p = 0.0799).
3.5 |. Social isolation and K+-induced extracellular DA levels in the NAc
To evaluate if a depolarizing stimulus of 70 mM K+ modified extracellular DA levels in the NAc of group-housed or single-housed rats, we performed microdialysis for approximately 100 min, collecting dialysates every 10 min. At minute 60, we infused K+-aCSF through the microdialysis probe for 10 min to induce depolarization (Figure 5b). A depolarizing K+ stimulus increased extracellular DA levels in single-housed and group-housed rats compared with their basal levels (2-way ANOVA, Table 1; Time p = 0.0001). The K+-induced DA release was also significantly different between groups (Table 1: Treatment: p = 0.0146; Interaction p = 0.0015; Table 2: Min 70, p < 0.05). (For complete statistical values, see Tables 1 and 2).
TABLE 2.
Sidak post-hoc comparisons of data analyzed by 2-way ANOVA (Figures 5b and 6b)
| Minute | Dopamine (Figure 5b) | Glutamate (Figure 6b) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Mean | ||||||||
| dif | t | Sign | Sum | dif | t | Sign | Sum | ||
| Single vs. group housed |
10 | −0.0498 | 0.053 | No | ns | −1.596 | 0.053 | No | ns |
| 20 | 10.46 | 0.124 | No | ns | 3.746 | 0.125 | No | ns | |
| 30 | −10.41 | 0.071 | No | ns | −2.150 | 0.072 | No | ns | |
| 40 | 4.382 | 0.104 | No | ns | −3.122 | 0.104 | No | ns | |
| 50 | 18.61 | 0.617 | No | ns | −18.37 | 0.617 | No | ns | |
| 60 | −13.73 | 0.270 | No | ns | −8.124 | 0.270 | No | ns | |
| 70 | −305.8 | 1.675 | Yes | **** | −50.32 | 1.675 | No | ns | |
| 80 | −85.58 | 0.412 | No | ns | −12.37 | 0.412 | No | ns | |
| 90 | −28.53 | 2.607 | No | ns | −78.32 | 2.607 | No | ns | |
| 100 | −6.562 | 0.805 | No | ns | −24.18 | 0.805 | No | ns | |
Timepoint 70 is significantly different between single-housed and group-housed rats.
3.6 |. CP-154,526 decreases DA levels in the NAc of isolated rats
To determine the modulatory role of CRF-R1 on extracellular DA levels in the NAc, two groups of animals were infused with the CRF-R1 antagonist, CP-154,526, through the microdialysis probe at minute 30 (Lemos et al., 2012). We analyzed the effect of CP-154,526 on extracellular DA levels by selecting the time points 40–100, which represent the time points when the antagonist was infused through the tissue. A 3-way MANOVA of time points 40–100 shows a significant interaction between housing and the CRF-R1 antagonist (p = 0.0330), indicating that housing conditions affect CRF-R1 modulation of extracellular DA in the NAc. Figure 5c and d shows that CP-154,526 decreased extracellular DA at all time points measured in single-housed rats, an effect not observed in group-housed rats.
Our results also indicate there is a significant difference across time: extracellular DA varied throughout the time period that it was collected. The infusion of K+ at min 70 induced an increase in extracellular DA in single- and group-housed animals with and without CP-154,526, which is reflected as a significant difference in the repeated measure variable (time; p = 0.0118). Data in Figure 5c and d were combined and analyzed with a 3-way repeated measures ANOVA (For statistical values, see Tables 2 and 3).
3.7 |. Social isolation increases, and CP-154,526 decreases, GLU release in the NAc
A 3-way repeated measures ANOVA was used to analyze extracellular GLU levels in the NAc shell using the data from Figure 6c and d. An analysis of time points 40–100 indicates that (a) housing conditions (Housing: p = 0.0129) affect extracellular GLU levels in the NAc, (b) extracellular GLU levels vary across the time points measured (Time: p = 0.0261) and (c) housing (single or grouped) affects CP-154,526 modulation of extracellular GLU in the NAc across time (Time × Housing; p = 0.0385). Importantly, a three-way interaction (Time × Housing × CP-154,526) was observed, indicating that the effect of CP-154,526 varies according to housing conditions (grouped or single) throughout time. In other words, only single-housed rats showed an increase in GLU after K+, an effect that was enhanced by CP-154,526. In contrast, group-housed rats did not show an increase in GLU in response to K+.
If we limit our comparisons to minutes 40–60, the data show an interaction between all variables (p = 0.0490), particularly an effect of housing (p = 0.0232). CP-154,526 shows a trend to decrease extracellular GLU only in group-housed animals, hinting the potential effects of CRF-R1 on GLU response (Housing × CP-154,526; p = 0.0903). Further studies are required to confirm the role of CRF-R1 on GLU release in the NAc. (For statistical values, see Tables 2 and 3).
3.8 |. Social isolation does not alter CRF levels in the NAc
On day 31, separate groups of single-and group-housed rats were euthanized, the NAc dissected and homogenized, and the supernatant analyzed for CRF by ELISA. CRF was normalized to the total amount of protein in the NAc of each rat. No significant differences were observed in CRF levels between group-housed and single-housed rats (Figure 7). The average amount of normalized CRF peptide in group-housed rats was 0.80 ± 0.08 (μg/mg), and for single-housed rats, it was 0.75 ± 0.03 (μg/mg). Data were analyzed by non-paired Student’s t test (t = 0.6303; p = 0.5517).
4 |. DISCUSSION
4.1 |. Summary
Recent studies show that psychiatric disorders during adulthood can be traced back to life adversities during infancy and early adolescence (Long Term Consequences of Child Abuse and Neglect, 2019). In rodents, post-weaning social isolation has been used to study the effects of neglect during early adolescence on social, emotional and addictive behaviors among others (Lukkes et al., 2009). Most studies socially isolate young males for 4–6 weeks and assess the consequences during early adulthood (Lukkes et al., 2009). We chose to investigate the impact of 10 days of social isolation immediately after weaning, to determine if the detrimental effects of isolation are present soon afterwards, during mid-adolescence, or if they require some time for the effects to be manifested.
Our main goal was to evaluate behavioral and neurochemical alterations in the NAc, induced by social isolation in adolescent male rats. The role of CRF-R1 in mediating these changes was also investigated. Our data confirm previous studies that report an increase in body weight, ambulation and anxiety-like behaviors in socially isolated rats. In the NAc, no differences in CRF content or in basal extracellular levels of DA or GLU were found between groups. Upon a depolarizing stimulus, there was a significant increase in extracellular DA levels in the NAc of both groups, with single-housed rats displaying a higher percent change than group-housed rats. Infusion of the CRF-R1 antagonist into the NAc increased the K+-induced percent change of extracellular DA in group-housed animals compared with aCSF-control infused animals. In single-housed rats, CRF-R1 reduced extracellular DA levels. These results suggest that in the NAc, CRF-R1 may be acting to mitigate the extracellular dopaminergic response to a depolarizing stimulus, as observed in group-housed rats, and that isolation overrides the moderating effect of CRF-R1 on stimulus-induced DA release. CRF-R1 also decreased extracellular GLU levels in group-housed rats, an effect not observed in socially isolated rats, suggesting that CRF-R1 plays an important role in regulating basal extracellular GLU in the NAc.
4.2 |. Social isolation increased anxiety
This study found that social isolation of adolescent rats increased anxiety-like behaviors when measured during adolescence. Anxiety was assessed as a decrease in the number of entries, and in the time spent, in the open arms of an EPM, and an increase in the time spent in the closed arms of an EPM (Lukkes et al., 2009). In addition, single-housed rats spent less time in the center of an open field arena, than grouped-housed rats. Our data indicate that the time spent in the open arms is more sensitive to isolation than the number of entries into the open arms, similar to what is observed with anxiogenic drugs (Pellow et al., 1985). These results also illustrate that the anxiogenic effects of isolation are independent of a possible sedative or depressive effect as the total amount of entries, and the distance travelled in the open field, is greater in isolated males. Previous studies have confirmed that social isolation after weaning exerts detrimental effects on emotion, cognition, hyperactivity and motivated behaviors (Begni et al., 2020; Lukkes et al., 2009). Indeed, changes in functional connectivity and gene expression, as well as the appearance of dysfunctional behaviors, have been observed (Begni et al., 2020). Increases in contextual fear memory deficits (Okada et al., 2014) and aggression (Matsumoto et al., 2005) have also been reported. Furthermore, animals that were socially isolated after weaning, stressed and tested afterwards as adults show an increase in anxiety (Ishikawa et al., 2015).
The current study extends these findings and shows that 10 days of social isolation is sufficient to disrupt emotional behaviors and indicates that the effects of isolation on anxiety can be observed as early as adolescence. Anxiety and stress share common neural substrates, such as the hypothalamus, amygdala, prefrontal cortex and striatum (Daviu et al., 2019; Mobbs et al., 2007). Thus, it is not surprising that isolating rats immediately after weaning can cause dysfunctions in brain areas that regulate emotion (Lemos et al., 2012; Okada et al., 2014). Children that experience a difficult childhood, due to abuse or isolation among others, are more susceptible to develop emotional and psychiatric disorders (Kapur et al., 2005). Indeed, a recent study calculated that approximately 50% of adult patients diagnosed with depression and anxiety were subjected to maltreatment during childhood and adolescence (Li et al., 2016).
4.3 |. Social isolation increased locomotor activity
Similar to other studies, we found that isolation increased ambulation in an open field as well as the number of arm entries in an EPM (Ieraci et al., 2016; Shao et al., 2009). Ambulation and navigation are complex behaviors that involve whole brain computation (Hughes & Celikel, 2019) and are dependent on the activation of sensorimotor circuits (Hughes & Celikel, 2019). Pre-pulse inhibition (PPI) is used as an operational measure of sensorimotor gating (Fitzgerald & Pickel, 2018; Kohl et al., 2013). Deficiencies in PPI are characteristic of schizophrenic patients and of other psychiatric conditions (Fitzgerald & Pickel, 2018; Kohl et al., 2013). Recent studies indicate that social isolation during adolescence induces deficits in PPI that lasts into adulthood (Wilkinson et al., 1994). Indeed, manipulation of DA and GLU levels in the NAc (Mumtaz et al., 2018), as well as rearing rats in isolation (Fone & Porkess, 2008; Wood et al., 2005), induces PPI deficits that may be related to some of the behavioral changes observed in socially isolated rats, such as increased anxiety, ambulation and a hyperactive HPA axis (Fitzgerald & Pickel, 2018).
4.4 |. Social isolation increased body weight
Body weight is intricately regulated by several interconnected brain, adipose and gut tissues, as well as by a plethora of peptides that drive food intake and energy expenditure. From hindbrain (nucleus of the tractus solitarius), to limbic (amygdala, hypothalamus, striatum) to cortical (prefrontal cortex) substrates, they all contribute to the homeostatic regulation of body weight. Hormones, particularly those from the thyroid, can also alter the metabolic rate. Previous studies have found that early life adversity can contribute to the development of obesity, metabolic disease and higher food intake (Koolhaas et al., 2010; Morris et al, 2014; Vargas et al., 2016), the effect depending on the type and duration of stress, as well as on individual reactivity to stress. Early life stress can initiate adaptive changes with long-lasting consequences. For example, increases in plasma glucocorticoid resulting from early life adversity can lead to a greater susceptibility to develop metabolic syndrome as adults and result in higher body weight (Morris et al, 2014; Vargas et al., 2016). Indeed, increases in body weight, fasting blood glucose and insulin levels are reported in rats after early life stress (Vargas et al., 2016).
In this study, we found that after 10 days of single housing, rats weighed significantly more than their group-housed counterparts, consistent with previous studies reporting that chronic stress, particularly during development, results in an increase in body weight and of food intake (Fiala et al., 1977; Nakhate et al., 2011; Vargas et al., 2016; Zaias et al., 2008). Nonetheless, there are studies that find an opposite effect, which may be partially explained by the day and length of the isolation procedure among other factors (Shao et al., 2009). Interestingly, the increase in body weight of socially isolated rats observed in our study occurred even though isolated animals show greater locomotor activity than group-housed rats. It is possible that the increased body weight may be related to the psychological and emotional effects induced by social isolation, such as increased anxiety, resulting in higher food intake, or by changes in metabolic rate (Fiala et al., 1977; Jahng & Houpt, 2001).
CRF is one of the main secretagogues during a stress response and has been implicated in the regulation of body weight, mainly by altering food intake and energy expenditure (Dedic et al., 2018; Wei et al, 2019). A recent study reports that dysregulation of CRF expression in the NAc may be correlated with compulsive eating behaviors (Wei et al., 2019). However, in our current study, we did not find that social isolation altered CRF levels in the NAc. Consistent with our results, socially isolating neonatal pups (PN 2–14) does not alter hypothalamic or hippocampal CRF levels when measured in adulthood (Husum & Mathe, 2002).
Considering unchanged expression of CRF in the NAc in the current study, one proposed scenario is that isolation may induce changes in CRF receptor density or affinity. Indeed, studies by Wei et al. (2019) show that chronic stress increased CRF1 and CRF2, as well as D2R, in the NAc of adult male mice. An additional scenario is that isolation may alter CRF expression (or receptor population) in other brain areas such as the prefrontal cortex, an important regulator of goal-directed behavior, such as food and drug reward, and of inhibition of inappropriate actions (Wise & Morales, 2010). Indeed, rats exposed to early life adversity attributed a greater incentive value to a reward cue (Hynes et al., 2017). Further studies are required to elucidate the mechanism involved.
4.5 |. Social isolation increased K+-induced extracellular DA release in the NAc
In the current study, we did not observe changes in basal extracellular DA levels in the NAc. However, the DA response to a depolarizing stimulus was exacerbated in single-housed compared with group-housed rats. Previous studies have found an association between the increase in anxiety observed in animals subjected to early life adversity and an increased DA responsiveness to reward (Brake et al., 2004; Kosten et al., 2005; Matthews et al., 1999; McArthur et al., 2007; Ploj et al., 2003). A study by Shao et al. (2009) with rats socially isolated from days 32–51 reports no difference in DA levels in the NAc at day 52 but observed an increase in DA content at day 66. Similarly, Karkhanis et al. (2019) report an increase in DA release in response to simulation in NAc slices of rats isolated during adolescence. Kapur et al. (2005) also find that dopaminergic neuronal firing becomes dysregulated after early life stress and suggest that this may be responsible for the “usurpation of salience and novelty” observed with increased anxiety. Our results confirm these earlier studies that report a “hyperresponsive dopaminergic system” as a result of early life adversity (Fulford & Marsden, 1998; Lapiz et al., 2003).
4.6 |. Blocking CRF-R1 increases extracellular DA release in the NAc but had no effect on socially isolated rats
Infusion of the CRF-R1 antagonist into the NAc increased the extracellular DA response induced by K+ in group-housed animals. In contrast, single-housed rats showed a decrease in extracellular DA levels after CP-154,526 infusion. These data show that CRF-R1 participates, at least partially, in regulating extracellular DA levels in the NAc. They also convey that a possible role of CRF-R1 in the NAc is to moderate the response of extracellular DA to a depolarizing stimulus.
The presence of CRF-R1 immunoreactivity in cell bodies and dopaminergic terminals of the NAc (Lemos et al., 2012) and the fact that pharmacological and genetic manipulation of the CRF-R1 population in the NAc alters the firing pattern and DA release of dopaminergic neurons (Lemos et al., 2012; Refojo et al., 2011) suggest a regulatory role of CRF-R1 upon mesocorticolimbic dopaminergic circuitry. CRF-R1 may limit the dopaminergic response to a depolarizing stimulus, and isolation appears to override this limitation, an indication of a dysregulated CRF-R1-dopaminergic system. Indeed, evidence suggests that DA release in the NAc is dependent on the activation of CRF receptors (Lemos et al., 2012). Studies by Refojo et al. (2011) propose that the subset of dopaminergic neurons that contain CRF-R1 is responsible for increasing firing of DA neurons in response to stress. Conditional mutagenesis experiments where CRF-R1 in DA neurons are rendered non-functional show an increase in action potential firing of DA neurons (Refojo et al., 2011), an effect similar to what was observed in group-housed rats following administration of the CRF-R1 antagonist. The presence of CRF-R1 on glutamatergic and dopaminergic neurons is suggested to play a dual role in the regulation of emotional behaviors, acting in an antagonistic fashion to regulate the adaptive responses to stress (Refojo et al., 2011). Indeed, rendering CRF-R1 non-functional in glutamatergic and in dopaminergic neurons cancels the effect on anxiety behaviors (Refojo et al., 2011). Other studies show that D2 receptors present in DA terminals of the NAc are increased by social isolation and antagonism of CRF-R1 in adult male rats reverses this effect (Djouma et al., 2006). Evidence for cholinergic modulation of DA in the NAc by activation of CRF-R1 has also been shown to play a role (Lemos et al., 2019).
Taken together, these data suggest a regulatory role of CRF-R1 on mesolimbic dopaminergic circuitry. Our future studies will evaluate CRF-R1 and CRF-R2 levels in the NAc and the modulatory role of CRF-R2 in mediating the effects of social isolation on DA levels in the NAc. The possible role of cholinergic interneurons and of accumbal D2 receptors in mediating dopaminergic dysregulation in socially isolated adolescent rodents will also be examined.
4.7 |. Social isolation did not affect basal extracellular glutamate release
Basal extracellular GLU levels in the NAc were not different between single- and group-housed rats. Other investigators also report that extracellular basal levels of GLU and DA are not affected under different isolation procedures (Grotewold et al., 2014; Howes et al., 2000; McCormick et al., 2002). In contrast, K+ increased extracellular GLU only in single-housed rats, indicating that isolation induces a hyperresponsive GLU response to a depolarizing stimulus.
4.8 |. Blocking CRF-R1 decreased extracellular GLU release in the NAc of group-housed rats
Blocking CRF-R1 receptors decreased extracellular GLU release in group-housed but not in single-housed rats. Thus, isolation overrides the effect of the CRF-R1 antagonist on the extracellular release of DA and GLU. Our statistical analysis reveals an interaction between Time, Housing and CP-154,526, indicating that they all contributed to modulate the release of GLU. Very few studies have evaluated the role of stress on NAc GLU levels. Recent studies indicate that glutamatergic input into the NAc regulates dendritic spine density as well as susceptibility to social stress (Christoffel et al., 2015). An increase in excitatory synaptic neurotransmission in the NAc with increased anxiety has also been reported (Heshmati et al., 2016).
The glutamatergic input to the NAc is diverse, with projections coming from the basolateral amygdala, prefrontal cortex, hippocampus and mediodorsal thalamus (for review see Britt et al., 2012; Xu et al., 2020). Activation of these pathways also elicits distinct behavioral and physiological effects (Britt et al., 2012). For example, optical stimulation of hippocampal neurons elicits the highest excitatory currents of postsynaptic neurons in the NAc compared with projections from the amygdala or prefrontal cortex (Britt et al., 2012). Glutamatergic neuronal populations may have intrinsic differences in input resistance, firing threshold and proximity to glial cells, among other factors, that ultimately may affect their reactivity to stress.
5 |. CONCLUSION
In summary, this study finds that isolating adolescent rats for 10 days after weaning increased anxiety and locomotor activity. Isolated rats also showed an increase in body weight that may be related to increased food consumption or decreased metabolism. No changes were observed in CRF content, or in basal extracellular DA or GLU, in the NAc. Interestingly, DA and GLU terminals in the NAc of isolated rats are hyperresponsive to a depolarizing stimulus compared with group-housed rats and may partially explain the increase in anxiety.
Blocking CRF-R1 receptors decreased extracellular DA in single-housed rats, an effect not observed in group-housed rats. CP-154,526 also decreased GLU in group-housed animals. Thus, our data show that CRF-R1 receptors modulate dopaminergic and glutamatergic tone in the NAc. Our results suggest that social isolation affects the CRF system altering dopaminergic and glutamatergic tone in the NAc, which in turn may affect emotionality. This study provides the first evidence of the detrimental effect of social isolation during adolescence on anxiety and suggests that dysregulation of the CRF system in the NAc may alter DA and GLU release in the NAc and contribute to the pathologies observed in adolescents exposed to early life adversity. Further studies are needed to elucidate the mechanism by which CRF-R1 regulates DA and GLU transmission in the NAc.
DATA AVAILABILITY STATEMENT
Data is available upon request and by consent of the principal investigators.
ACKNOWLEDGEMENTS
This work was supported by Grant #1545803 of the Program for International Research and Education (PIRE) of the Office for International Research and Education (OIRE) from the National Science Foundation to ACS and by FONDECYT grant nos. 1150244 and 1191274, of the Ministry of Education of Chile to KG. EUPC was supported by the University of Puerto Rico, Medical Sciences Campus, Research Initiative for Scientific Enhancement (RISE) Program under the National Institutes of Health (NIH) grant R25 GM061838.
Funding information
National Institutes of Health, Grant/Award Number: RISE R25 GM061838; Ministry of Education, Chile, Grant/Award Number: 1191274; National Science Foundation USA, Grant/Award Number: OISE PIRE #1545803; Fondo Nacional de Desarrollo Científico y Tecnológico, Grant/Award Number: 1150244
Abbreviations:
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- CP-154,526
antagonist of CRF-R1
- CRF
corticotropin releasing factor
- CRF-R1
corticotropin releasing factor receptor type 1
- CRF-R2
corticotropin releasing factor receptor type 2
- CRH
corticotropin releasing hormone
- D2
dopamine type 2 receptor
- DA
dopamine
- DR
dorsal raphe
- ELISA
enzyme-linked immunosorbent assay
- EPM
elevated plus maze
- GLU
Glutamate
- NAc
nucleus accumbens
- OF
open field
- PFC
prefrontal cortex
- PN
postnatal
- PPI
pre-pulse inhibition
- VTA
ventral tegmental area
Footnotes
CONFLICT OF INTEREST
None of the authors have any financial interests associated with the results herein submitted nor with any company that could financially benefit from the results reported in this manuscript.
REFERENCES
- Agid O, Kohn Y, & Lerer B (2000). Environmental stress and psychiatric illness. Biomedicine & Pharmacotherapy, 54, 135–141. 10.1016/S0753-3322(00)89046-0 [DOI] [PubMed] [Google Scholar]
- Backström T, & Winberg S (2013). Central corticotropin releasing factor and social stress. Frontiers in Neuroscience, 7, 117. 10.3389/fnins.2013.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begni V, Sanson A, Pfeiffer N, Brandwein C, Inta D, Talbot SR, Riva MA, Gass P, & Mallien AS (2020). Social isolation in rats: Effects on animal welfare and molecular markers for neuroplasticity. PLoS One, 15(10), e0240439. 10.1371/journal.pone.0240439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2007). The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology, 191, 391–431. 10.1007/s00213-006-0578-x [DOI] [PubMed] [Google Scholar]
- Biedermann SV, Biedermann DG, Wenzlaff F, Kurjak T, Nouri S, Auer MK, Wiedemann K, Briken P, Haaker J, Lonsdorf TB, & Fuss J (2017). An elevated plus-maze in mixed reality for studying human anxiety-related behavior. BMC Biology, 15, 125. 10.1186/s12915-017-0463-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, & Gratton A (2004). Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. European Journal of Neuroscience, 19, 1863–1874. 10.1111/j.1460-9568.2004.03286.x [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, & Bonci A (2012). Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron, 76(4), 790–803. 10.1016/j.neuron.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, & Miczek KA (2014). Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology, 231, 1557–1580. 10.1007/s00213-013-3369-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, Dey A, Smith M, Rebusi N, Pfau M, Ables JL, Aleyasin H, Khibnik LA, Hodes GE, Ben-Dor GA, Deisseroth K, Shapiro ML, Malenka RC, Ibanez-Tallon I, Han M-H, & Russo SJ (2015). Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nature Neuroscience, 18(7), 962–964. 10.1038/nn.4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long term consequences of child abuse and neglect, National Clearinghouse on Child Abuse and Neglect (2019)Long term consequences of child abuse and neglect, National Clearinghouse on Child Abuse and Neglect (April, 2019)
- Daviu N, Bruchas MR, Moghaddam B, Sandi C, & Beyeler A (2019). Neurobiological links between stress and anxiety. Neurobiology of Stress, 11, 100191. 10.1016/j.ynstr.2019.100191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedic N, Chen A, & Deussing JM (2018). The CRF family of neuropeptides and their receptors—Mediators of the central stress response. Current Molecular Pharmacology, 11, 4–31. 10.2174/1874467210666170302104053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing JM, Breu J, Kuhne C, Kallnik M, Bunck M, Glasl L, Yen YC, Schmidt MV, Zurmuhlen R, Vogl AM, Gailus Durner V, Fuchs H, Holter SM, Wotjak CT, Landgraf R, de Angelis MH, Holsboer F, & Wurst W (2010). Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. Journal of Neuroscience, 30, 9103–9116. 10.1523/JNEUROSCI.1049-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing JM, & Chen A (2018). The corticotropin-releasing factor family: Physiology of the stress response. Physiological Reviews, 98, 2225–2286. 10.1152/physrev.00042.2017 [DOI] [PubMed] [Google Scholar]
- Deutch AY, & Cameron DS (1992). Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience, 46, 49–56. [DOI] [PubMed] [Google Scholar]
- DeVries AC (2002). Interaction among social environment, the hypothalamic–pituitary–adrenal axis, and behavior. Hormones and Behavior, 41, 405–413. 10.1006/hbeh.2002.1780 [DOI] [PubMed] [Google Scholar]
- Djouma E, Card K, Lodge DJ, & Lawrence AJ (2006). The CRF1 receptor antagonist, antalarmin, reverses isolation-induced up-regulation of dopamine D2 receptors in the amygdala and nucleus accumbens of fawn-hooded rats. European Journal of Neuroscience, 23, 3319–3327. [DOI] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, & Chen A (2010). Resilience to social stress coincides with functional DNA methylation of the CRF gene in adult mice. Nature Neuroscience, 13, 1351–1353. 10.1038/nn.2642 [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, & Hardin M (2006). Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine, 36, 299–312. 10.1017/S0033291705005891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala B, Snow FM, & Greenough WT (1977). “Impoverished” rats weigh more than “Enriched” rats because they eat more. Developmental Psychobiology, 10, 537–541. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, & Pickel VM (2018). Adolescent isolation rearing produces a prepulse inhibition deficit correlated with expression of the NMDA GluN1 subunit in the nucleus accumbens. Brain Structure and Function, 223, 3169–3181. 10.1007/s00429-018-1673-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KCF, & Porkess MV (2008). Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neuroscience and Biobehavioral Reviews, 32, 1087–1102. 10.1016/j.neubiorev.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Fulford AJ, & Marsden CA (1998). Effect of isolation-rearing on conditioned dopamine release in vivo in the nucleus accumbens of the rat. Journal of Neurochemistry, 70, 384–390. [DOI] [PubMed] [Google Scholar]
- Girault JA, Barbeito L, Spampinato U, Gozlan H, Glowinski J,& Besson MJ (1986). In vivo release of endogenous amino acids from the rat striatum: Further evidence for a role of glutamate and aspartate in corticostriatal neurotransmission. Journal of Neurochemistry, 47, 98–106. 10.1111/j.1471-4159.1986.tb02836.x [DOI] [PubMed] [Google Scholar]
- Grotewold SK, Wall VL, Goodell DJ, Hayter C, & Bland ST (2014). Effects of cocaine combined with a social cue on conditioned place preference and nucleus accumbens monoamines after isolation rearing in rats. Psychopharmacology, 231, 3041–3053. 10.1007/s00213-014-3470-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CS (1934). Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. Journal of Comparative Psychology, 18, 385–403. 10.1037/h0071444 [DOI] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA,& Dautzenberg FM (2009). Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Annals of the New York Academy of Sciences, 1179, 120–143. 10.1111/j.1749-6632.2009.05011.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, Deussing JM, & Chen A (2016). Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nature Reviews Neuroscience, 17, 636–651. 10.1038/nrn.2016.94 [DOI] [PubMed] [Google Scholar]
- Heshmati M, Golden SA, Pfau ML, Christoffel DJ, Seeley EL, Cahill ME, Khibnik LA, & Russo SJ (2016). Mefloquine in the nucleus accumbens promotes social avoidance and anxiety-like behavior in mice. Neuropharmacology, 101, 351–357. 10.1016/j.neuropharm.2015.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan MR, Kalin NH, & Kelley AE (1997). Microinfusion of corticotropin-releasing factor into the nucleus accumbens shell results in increased behavioral arousal and oral motor activity. Psychopharmacology, 130, 189–196. 10.1007/s002130050228 [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, & Everitt BJ (2000). Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: Relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology, 151, 55–63. 10.1007/s002130000451 [DOI] [PubMed] [Google Scholar]
- Hughes S, & Celikel T (2019). Prominent inhibitory projections guide sensorimotor computation: An invertebrate perspective. BioEssays, 41, e1900088. 10.1002/bies.201900088 [DOI] [PubMed] [Google Scholar]
- Husum H, & Mathe AA (2002). Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology, 27, 756–764. 10.1016/S0893-133X(02)00363-9 [DOI] [PubMed] [Google Scholar]
- Ieraci A, Mallei A, & Popoli M (2016). Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plasticity, 2016, 6212983. 10.1155/2016/6212983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa J, Nishimura R, & Ishikawa A (2015). Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats. European Journal of Neuroscience, 41, 442–453. 10.1111/ejn.12825 [DOI] [PubMed] [Google Scholar]
- Itoga CA, Chen Y, Fateri C, Echeverry PA, Lai JM, Delgado J, Badhon S, Short A, Baram TZ, & Xu X (2019). New viral-genetic mapping uncovers an enrichment of corticotropin-releasing hormone-expressing neuronal inputs to the nucleus accumbens from stress-related brain regions. The Journal of Comparative Neurology, 527, 2474–2487. 10.1002/cne.24676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng JW, & Houpt TA (2001). MK801 increases feeding and decreases drinking in nondeprived, freely feeding rats. Pharmacology, Biochemistry and Behavior, 68, 181–186. 10.1016/S0091-3057(00)00434-2 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, & Duffy P (1995). Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Research, 675(1–2), 325–328. 10.1016/0006-8993(95)00013-G [DOI] [PubMed] [Google Scholar]
- Kapur S, Mizrahi R, & Li M (2005). From dopamine to salience to psychosis—Linking biology, pharmacology and phenomenology of psychosis. Schizophrenia Research, 79, 59–68. 10.1016/j.schres.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Karkhanis AN, Leach AC, Yorgason JT, Uneri A, Barth S, Niere F, Alexander NJ, Weiner JL, McCool BA, Raab Graham KF, Ferris MJ, & Jones SR (2019). Chronic social isolation stress during peri-adolescence alters presynaptic dopamine terminal dynamics via augmentation in accumbal dopamine availability. ACS Chemical Neuroscience, 10, 2033–2044. 10.1021/acschemneuro.8b00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S, Heekeren K, Klosterkotter J, & Kuhn J (2013). Prepulse inhibition in psychiatric disorders apart from schizophrenia. Journal of Psychiatric Research, 47, 445–452. 10.1016/j.jpsychires.2012.11.018 [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Coppens CM, & Buwalda B (2010). Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Frontiers neuroendocrinology, 31(3), 307–321. 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, & Kehoe P (2005). Neurochemical and behavioral responses to cocaine in adult male rats with neonatal isolation experience. Journal of Pharmacology and Experimental Therapeutics, 314, 661–667. 10.1124/jpet.104.081216 [DOI] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, & Nemeroff CB (1996). Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology, 137, 1212–1218. 10.1210/endo.137.4.8625891 [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, & Marsden CA (2003). Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neuroscience and Behavioral Physiology, 33, 13–29. [DOI] [PubMed] [Google Scholar]
- Lazarus R, & Cohen J (1977). Environmental stress. In Altman I, & Wohlwill JF (Eds.), Human behavior and environment: Advances in theory and research, 1st ed. (pp. 89–127). Plenum Press. [Google Scholar]
- Lemos JC, Shin JH, & Alvarez VA (2019). Striatal cholinergic interneurons are a novel target of corticotropin releasing factor. Journal of Neuroscience, 39, 5647–5661. 10.1523/JNEUROSCI.0479-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, & Phillips PE (2012). Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature, 490, 402–406. 10.1038/nature11436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, D’Arcy C, & Meng X (2016). Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: Systematic review, meta-analysis, and proportional attributable fractions. Psychological Medicine, 46, 717–730. 10.1017/S0033291715002743 [DOI] [PubMed] [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, & Young LJ (2007). CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Hormones and Behavior, 51, 508–515. 10.1016/j.yhbeh.2007.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Forster GL, Renner KJ, & Summers CH (2008). Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphé differentially affect serotonin release in the nucleus accumbens. European Journal of Pharmacology, 578, 185–193. 10.1016/j.ejphar.2007.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, & Forster GL (2009). Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Frontiers in Behavioural Neurosciences, 3, 18. 10.3389/neuro.08.018.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Pinna G, Puia G, Guidotti A, & Costa E (2005). Social isolation stress-induced aggression in mice: A model to study the pharmacology of neurosteroidogenesis. Stress, 8, 85–93. 10.1080/10253890500159022 [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW, Everitt BJ, & Caine SB (1999). Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology, 141, 123–134. 10.1007/s002130050816 [DOI] [PubMed] [Google Scholar]
- McArthur S, McHale E, & Gillies GE (2007). The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex-, region- and time-specific manner. Neuropsychopharmacology, 32, 1462–1476. 10.1038/sj.npp.1301277 [DOI] [PubMed] [Google Scholar]
- McCormick CM, Kehoe P, Mallinson K, Cecchi L, & Frye CA (2002). Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacology, Biochemistry and Behavior, 73, 77–85. 10.1016/S0091-3057(02)00758-X [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, & Frith CD (2007). When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science, 317, 1079–1083. 10.1126/science.1144298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Roth RH, & Bunney BS (1990). Characterization of dopamine release in the rat medial prefrontal cortex as assessed by in vivo microdialysis: Comparison to the striatum. Neuroscience, 36, 669–676. 10.1016/0306-4522(90)90009-S [DOI] [PubMed] [Google Scholar]
- Morales I, & Berridge KC (2020). ‘Liking’ and ‘wanting’ in eating and food reward: Brain mechanisms and clinical implications. Physiology & Behavior, 227, 113152. 10.1016/j.physbeh.2020.113152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MJ, Beilharz JE, Maniam J, Reichert AC, & Westbrook RF (2014). Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Journal of Neuroscience Biobehavioral Reviews, 58, 36–45. [DOI] [PubMed] [Google Scholar]
- Mumtaz F, Khan MI, Zubair M, & Dehpour AR (2018). Neurobiology and consequences of social isolation stress in animal model—A comprehensive review. Biomedicine & Pharmacotherapy, 105, 1205–1222. 10.1016/j.biopha.2018.05.086 [DOI] [PubMed] [Google Scholar]
- Mushtaq R, Shoib S, Shah T, & Mushtaq S (2014). Relationship between loneliness, psychiatric disorders and physical health ? A review on the psychological aspects of loneliness. Journal of Clinical and Diagnostic Research, 8, WE01–WE04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhate KT, Kokare DM, Singru PS, & Subhedar NK (2011). Central regulation of feeding behavior during social isolation of rat: Evidence for the role of endogenous CART system. International Journal of Obesity, 35, 773–784. 10.1038/ijo.2010.231 [DOI] [PubMed] [Google Scholar]
- Okada R, Matsumoto K, Tsushima R, Fujiwara H, & Tsuneyama K (2014). Social isolation stress-induced fear memory deficit is mediated by down-regulated neuro-signaling system and Egr-1 expression in the brain. Neurochemical Research, 39, 875–882. 10.1007/s11064-014-1283-5 [DOI] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (2005). The rat brain in stereotaxic coordinates, compact (6th edn.). New York: Academic Press. [Google Scholar]
- Pellow S, Chopin P, File SE, & Briley M (1985). Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods, 14, 149–167. 10.1016/0165-0270(85)90031-7 [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, & Nylander I (2003). Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience, 121, 787–799. 10.1016/S0306-4522(03)00499-8 [DOI] [PubMed] [Google Scholar]
- Prut L, & Belzung C (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. European Journal of Pharmacology, 463, 3–33. 10.1016/S0014-2999(03)01272-X [DOI] [PubMed] [Google Scholar]
- Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schutz G, Nave K-A, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, & Deussing JM (2011). Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science, 333, 1903–1907. 10.1126/science.1202107 [DOI] [PubMed] [Google Scholar]
- Ritchie H, & Roser M (2019). Mental health. Available at: https://ourworldindata.org/mental-health
- Robbins TW, Jones GH, & Wilkinson LS (1996). Behavioural and neurochemical effects of early social deprivation in the rat. Journal of Psychopharmacology, 10, 39–47. 10.1177/026988119601000107 [DOI] [PubMed] [Google Scholar]
- Seibenhener ML, & Wooten MC (2015). Use of the open field maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments, 96, e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao F, Jin J, Meng Q, Liu M, Xie X, Lin W, & Wang W (2009). Pubertal isolation alters latent inhibition and DA in nucleus accumbens of adult rats. Physiology & Behavior, 98, 251–257. 10.1016/j.physbeh.2009.05.021 [DOI] [PubMed] [Google Scholar]
- Skutella T, Probst JC, Renner U, Holsboer F, & Behl C (1998). Corticotropin-releasing hormone receptor (type I) antisense targeting reduces anxiety. Neuroscience, 85, 795–805. 10.1016/S0306-4522(97)00682-9 [DOI] [PubMed] [Google Scholar]
- Slater PG, Yarur HE, & Gysling K (2016). Corticotropin-releasing factor receptors and their interacting proteins: Functional consequences. Molecular Pharmacology, 90, 627–632. 10.1124/mol.116.104927 [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, & Casey BJ (2010). A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition, 72, 124–133. 10.1016/j.bandc.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor-Zarate R, Abarca J, Araya KA, Renard GM, Andres ME, & Gysling K (2015). Exposure to repeated immobilization stress inhibits cocaine-induced increase in dopamine extracellular levels in the rat ventral tegmental area. Pharmacological Research, 101, 116–123. 10.1016/j.phrs.2015.08.015 [DOI] [PubMed] [Google Scholar]
- Stevens A, & White A (2010). ACTH: Cellular peptide hormone synthesis and secretory pathways. Results and Problems in Cell Differentiation, 50, 63–84. [DOI] [PubMed] [Google Scholar]
- Treit D, & Fundytus M (1988). Thigmotaxis as a test for anxiolytic activity in rats. Pharmacology, Biochemistry and Behavior, 31, 959–962. 10.1016/0091-3057(88)90413-3 [DOI] [PubMed] [Google Scholar]
- Vargas J, Junco M, Gomez C, & Lajud N (2016). Early life stress increases metabolic risk, HPA axis reactivity, and depressive-like behavior when combined with postweaning social isolation in rats. PLoS One, 11, e0162665. 10.1371/journal.pone.0162665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iñiguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolaños CA, Barrot M, McClung CA, & Nestler EJ (2009). CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nature Neuroscience, 12(2), 200–209. 10.1038/nn.2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei NL, Quan Z-F, Zhao T, Yu X-D, Xie Q, Zeng J, Ma FK, Wang F, Tang QS, Wu H, & Zhu JH (2019). Chronic stress increases susceptibility to food addiction by increasing the levels of DR2 and MOR in the nucleus accumbens. Neuropsychiatric Disease and Treatment, 15, 1211–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, & Feldon J (2004). Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behavioral Brain Research, 152, 279–295. 10.1016/j.bbr.2003.10.015 [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Killcross SS, Humby T, Hall FS, Geyer MA, & Robbins TW (1994). Social isolation in the rat produces developmentally specific deficits in prepulse inhibition of the acoustic startle response without disrupting latent inhibition. Neuropsychopharmacology, 10, 61–72. 10.1038/npp.1994.8 [DOI] [PubMed] [Google Scholar]
- Williams CL, Buchta WC, & Riegel AC (2014). CRF-R2 and the heterosynaptic regulation of VTA glutamate during reinstatement of cocaine seeking. Journal of Neuroscience, 34, 10402–10414. 10.1523/JNEUROSCI.0911-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, & Morales M (2010). A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Research, 16(1314), 38–43. 10.1016/j.brainres.2009.09.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DA, Buse JE, Wellman CL, & Rebec GV (2005). Differential environmental exposure alters NMDA but not AMPA receptor subunit expression in nucleus accumbens core and shell. Brain Research, 1042, 176–183. 10.1016/j.brainres.2005.02.029 [DOI] [PubMed] [Google Scholar]
- Xu L, Nan J, & Lan Y (2020). The nucleus accumbens: A common target in the comorbidity of depression and addiction. Frontiers in Neural Circuits, 14, 37. 10.3389/fncir.2020.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Dai J, Liu C, Chen Y, Xin F, Zhou F, Zhou X, Huang Y, Wang J, Zou Z, Li J, Ebstein RP, Kendrick KM, Zhou B, & Becker B (2019). Common and disorder-specific neurofunctional markers of dysregulated empathic reactivity in major depression and generalized anxiety disorder. Psychotherapy and Psychosomatics, 89, 114–116. 10.1159/000504180 [DOI] [PubMed] [Google Scholar]
- Zahm DS (1999). Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Annals of the New York Academy of Sciences, 877, 113–128. 10.1111/j.1749-6632.1999.tb09264.x [DOI] [PubMed] [Google Scholar]
- Zaias J, Queeney TJ, Kelley JB, Zakharova ES, & Izenwasser S (2008). Social and physical environmental enrichment differentially affect growth and activity of preadolescent and adolescent male rats. Journal of the American Association for Laboratory Animal Science, 47, 30–34. [PMC free article] [PubMed] [Google Scholar]
- Zhou J-N, & Fang H (2018). Transcriptional regulation of corticotropin-releasing hormone gene in stress response. IBRO Reports, 5, 137–146. 10.1016/j.ibror.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar I, & Weinstock M (2011). Differential effect of prenatal stress on the expression of cortiocotrophin-releasing hormone and its receptors in the hypothalamus and amygdala in male and female rats. Journal of Neuroendocrinology, 23, 320–328. 10.1111/j.1365-2826.2011.02117.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request and by consent of the principal investigators.


