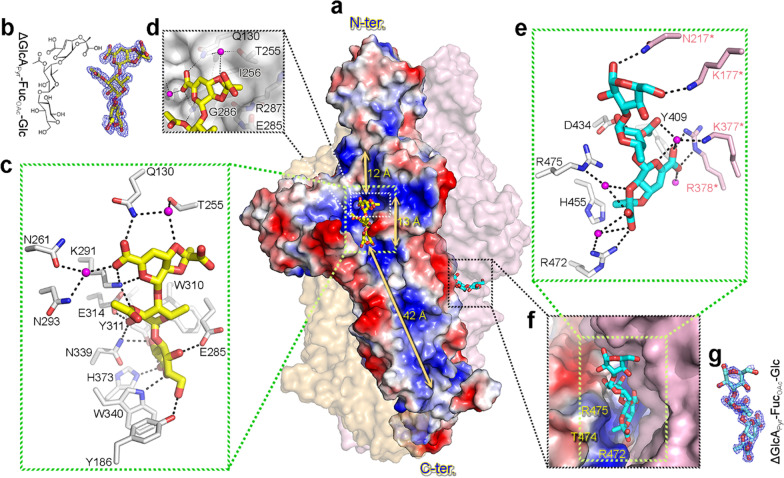
Fig. 4.
Two distinct carbohydrate-binding sites with bound trisaccharides. a Surface representation of the structure of trisaccharide-bound K1 lyase, with a view the same as in Fig. 2d. The surface charge potentials of the subunit are shown, with blue and red color representing the positive and negative charge, respectively. The lengths for different regions of the solvent-accessible groove are indicated. b The chemical structure and final refined model of the bound trisaccharide at the solvent-accessible groove of a subunit. The 1σ 2Fo–Fc omit map around the refined model is also shown. Abbreviations: ΔGlcAPyr = [2,3-(S)-pyruvate]-β-D-∆4,5-GlcpA; FucOAc = O-acetyl-α-L-Fucp. c Detailed interaction of the bound trisaccharide with the enzyme at the solvent-accessible groove. d The pyruvyl group of the bound trisaccharide is docked to a small pocket in the solvent-accessible groove. e Detailed interaction of the bound trisaccharide with the enzyme at the inter-subunit pocket. f The pyruvyl group is docked to a deep pocket created by residues Arg472, Thr474 and Arg475. g The 1σ 2Fo–Fc omit map around the refined model of a bound trisaccharide at the inter-subunit pocket