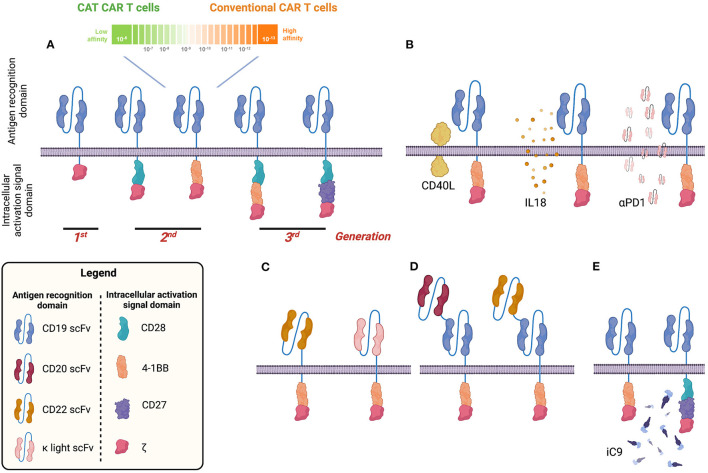
Figure 1.
Visual summary of different chimeric antigen receptor (CAR) designs to target B-cell malignancies. CD19 CAR has been tested in many clinical trials so far. Different generations of CD19 CAR have been developed including a second-generation CD19 CAR with low affinity for the CD19 antigen (CAT CAR) (A). Several groups proposed strategies to improve the long-term efficacy of the CD19 CAR by armoured CAR constructs capable of expressing both CD19 CAR and other molecules such as CD40L, interleukin 18 (IL-18), or programmed death 1 (PD1) capable of improving cytotoxic activity, reducing the exhaustion profile and sustaining the proliferation and persistence of CAR T cells (B). In addition to CD19, other B-cell antigens have been investigated and CARs have been generated and tested in preclinical and early-stage human clinical studies (e.g., CD22 and κ light chain) (C). To avoid tumour escape, bispecific CARs have been developed targeting, for example, CD19 and CD20 or CD22 (D). To improve the safety profile and generate a tool to mitigate/abrogate side effects, a suicide gene based on an inducible caspase 9 (iC9) has been developed and validated (E). Image created with BioRender.com.