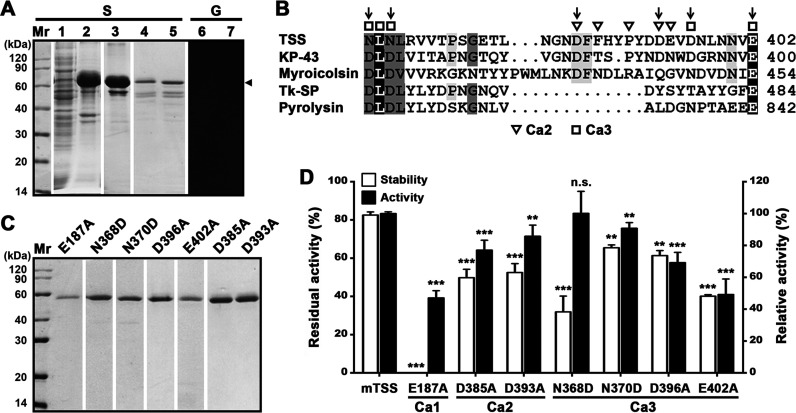
FIG 4.
Effects of the βJR domain and the Ca2+-binding sites on folding, stability, and activity of TSS. (A) Production and renaturation of ΔβP. The soluble (lane 1) and insoluble (lane 2) fractions of the cell lysate of E. coli producing ΔβP were separated by centrifugation. The recombinant ΔβP in the insoluble fraction was solubilized in 8 M urea and purified (lane 3), followed by renaturation to recover soluble ΔβP (lanes 4 to 7). The samples before (lanes 5 and 7) or after (lanes 1 to 4, and 6) TCA treatment were subjected to SDS-PAGE (S), followed by gelatin overlay assay (G). An arrowhead indicates the position of ΔβP on the gel. (B) Alignment of amino acid sequence around the Ca2+-binding sites (Ca2 and Ca3) in the βJR domain of TSS with those of KP-43 (AB051423), myroicolsin (AEC33275), Tk-SP subtilisin (Q5JIZ5), and pyrolysin (AAB09761). The residues serving as Ca2+-binding ligands are marked. Vertical arrows indicate the residues chosen for mutational analysis. (C) SDS-PAGE analysis of purified mature forms of the variants. (D) Comparison of the activities and thermostabilities of mTSS and its Ca2+-binding site variants. The azocaseinolytic activities were determined at 60°C in buffer B, and the relative activity was calculated with the activity of mTSS defined as 100%. For thermostability assay, the enzyme (2.5 μg/mL) was incubated at 70°C in buffer B for 3 h and then subjected to azocaseinolytic activity assay at 60°C. The residual activity is expressed as a percentage of the initial activity. The values are expressed as means ± SDs from three independent experiments (**, P < 0.01; ***, P < 0.001; n.s., no significance).