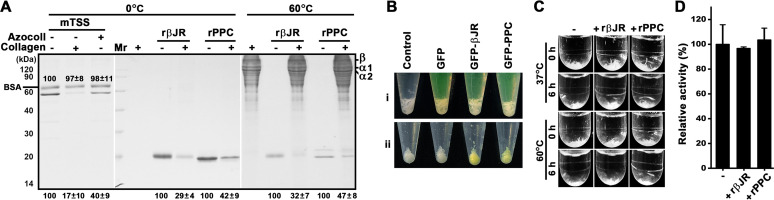
FIG 8.
The collagen-binding and collagen-swelling capacities of TSS and its βJR and PPC domains. (A) Collagen-binding capacity. The proteins (10 μg/mL) were incubated at 0°C or 60°C for 1 h in 100 μL of buffer B in the absence (−) or presence (+) of 3 mg of type I collagen or azocoll. In some cases, BSA (10 μg/mL) was added into the binding mixture as a control. After centrifugation, the supernatants were subjected to SDS-PAGE analysis. The loading volume of the sample incubated at 60°C was one-fourth of that incubated at 0°C. Numbers within or at the bottom of the gels are the densitometric ratios of each band compared with that of the control lane (in the absence of the substrate). The bands of α1, α2, and β chains of collagen are indicated. (B) Observation of the collagen-binding ability of GFP, GFP-βJR, and GFP-PPC. Type I collagen (3 mg) was suspended in 600 μL of buffer B without (control) or with 15 μM each protein (i) and then incubated at 60°C for 1 h with shaking. After centrifugation, the insoluble fraction was washed with buffer B three times and then photographically recorded (ii). (C) Collagen-swelling capacity. Type I collagen (3 mg) was suspended in 1 mL buffer B without (−) or with (+) 20 μg/mL of rβJR or rPPC. Before (0 h) or after incubation at 37°C or 60°C for 6 h with shaking, the samples were photographically recorded. (D) Activities of mTSS against rβJR- or rPPC-treated type I collagen. Type I collagen was heated at 60°C in the absence (−) or presence (+) of rβJR or rPPC as described in (B), recovered by centrifugation, and then used as the substrate for measuring the activity of mTSS at 60°C in buffer B. The values are expressed as means ± SDs from three independent experiments (A, C).